��Ŀ����
14��ij�о���ѧϰС��������ϵ�֪��Ư�������ᷴӦ�����Ƶ���������ѧ����ʽΪ��Ca��ClO��2+CaCl2+2H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$2CaSO4+2Cl2��+2H2O�������������ͼ1��ȡ��������֤�����ʵ�ʵ�飮�Իش���1����ʵ����A���ֵ�װ����ͼ2��b����дװ�õ���ţ���
��2���������һ��ʵ�飬֤��ϴ��ƿC�е�Na2SO3�Ѿ�������������ʵ�鲽�裩��ȡ������Ӧ�����Һ���Թ��У�����HCl��Һ�����ٲ�������Ϊֹ���ٵμ�BaCl2��Һ������а�ɫ�������ɣ�֤��Na2SO3�ѱ�������
��3��д��Dװ���з�����Ӧ�����ӷ���ʽHCO3-+H+�TH2O+CO2����
��4����С���ֽ���������ʵ�飺��ȡƯ��2.0g����ĥ���ܽ⣬���Ƴ�250ml ��Һ��ȡ25ml ���뵽��ƿ�У��ټ��������KI��Һ������H2SO4��Һ�����ã�����ȫ��Ӧ����0.1mol/L��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ��2Na2S2O3+I2=Na2S4O6+2NaI��Ӧ���ʱ������ȥNa2S2O3 20.0ml�����Ư����Ca��ClO��2����������Ϊ��35.75%��
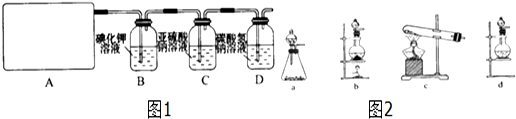
���� ��1�����ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã�
��2��������ǿ�����ԣ�������������л�ԭ�ԣ�����������������������ܷ���������ԭ��Ӧ������������ӡ������Ӻ������ӣ�����������Ʊ����������������ƣ�������������ӵļ��鷽�����鼴�ɣ�
��3��������ˮ��Ӧ��������ʹ����ᣬ������̼�����Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼��
��4���ȸ��ݴ�����ƺ���������ƵĹ�ϵʽ���������Ƶ�����������������������ʽ���㼴�ɣ�
��� �⣺��1������Ca��ClO��2+CaCl2+2H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$2CaSO4+2Cl2��+2H2O��֪����Ӧ��Ϊ������Һ�壬��Ӧ����Ϊ���ȣ�����ѡ��װ��b��
�ʴ�Ϊ��b��
��2��������ǿ�����ԣ�������������л�ԭ�ԣ�����������������������ܷ���������ԭ��Ӧ������������ӡ������Ӻ�������Cl2+SO32-+H2O=SO42-+2Cl-+2H+������������Ʊ������������������ƣ������ƺ��Ȼ����ܷ��� ��Ӧ���ɰ�ɫ�������ᱵ�������ᱵҲ�dz���������Ҫ���ų��������εĸ��ţ������Ȼ���������������ӣ����鷽��Ϊȡ������Ӧ�����Һ���Թ��У�����HCl��Һ�����ٲ�������Ϊֹ���ٵμ�BaCl2��Һ������а�ɫ�������ɣ�֤��Na2SO3�ѱ�������
�ʴ�Ϊ��ȡ������Ӧ�����Һ���Թ��У�����HCl��Һ�����ٲ�������Ϊֹ���ٵμ�BaCl2��Һ������а�ɫ�������ɣ�֤��Na2SO3�ѱ�������
��3��������ˮ��Ӧ��������ʹ����ᣬ������̼�����Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ӷ���ʽΪ��HCO3-+H+�TH2O+CO2����
�ʴ�Ϊ��HCO3-+H+�TH2O+CO2����
��4�����ݷ�Ӧ��֪���ڣ�Ca��ClO��2��2Cl2��2I2��4Na2S2O3��
n[Ca��ClO��2]=$\frac{1}{4}$n��Na2S2O3��=20.0 mL��10-3 L•mL-1��0.1 mol•L-1��$\frac{250ml}{25ml}$=0.005 mol��
Ca��ClO��2%=$\frac{0.005mol��143g/mol}{2.0g}$��100%=35.75%��
�ʴ�Ϊ��35.75%��
���� ���⿼����������ʵ�����Ʊ������ʵļ��飬��ȷ�Ʊ�ԭ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | �٢ڢ� | B�� | �ڢܢ� | C�� | ���٢� | D�� | �٢ڢۢܢ� |
| A�� | �κδ����ﶼ����һ��Ԫ����ɵ� | |
| B�� | һ��Ԫ��ֻ�����һ�ֵ��� | |
| C�� | �κ�һ�ֻ����ﶼ���ɲ�ͬ��Ԫ����ɵ� | |
| D�� | �κ����ʶ����ɷ��ӹ��ɵ� |
| A�� | ԭ�������ֻ���������ӵ�Ԫ�ض��ڵڢ�A�� | |
| B�� | ����ͨ���ڹ���Ԫ����Ѱ�Ҵ��������¡���ʴ�ĺϽ���� | |
| C�� | ���ۻ������п��ܺ������Ӽ� | |
| D�� | ˮ��Һ�ܵ���Ļ����ﶼ�����ӻ����� |
 ʵ��������ͼ��ʾװ����ȡ��ϩ��
ʵ��������ͼ��ʾװ����ȡ��ϩ��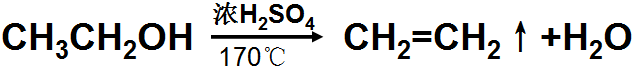
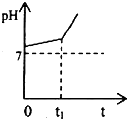 ���û�ѧ��Ӧԭ���о���Һ����������ʾ�����Ҫ���壮��ش��������⣺
���û�ѧ��Ӧԭ���о���Һ����������ʾ�����Ҫ���壮��ش��������⣺