��Ŀ����
11��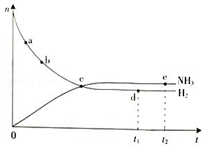 �ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����壮������ѧ֪ʶ�ش��������⣺
�ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����壮������ѧ֪ʶ�ش��������⣺��1�������ܱ������еķ�Ӧ��N2��g��+3H2��g���T2NH3��g����H��0��673K��30MPa��n��NH3����n��H2����ʱ��仯�Ĺ�ϵ��ͼ��ʾ��
����������ȷ����AD����ѡ���
A����a������Ӧ���ʱȵ�b�Ĵ�
B����c����Ӧ�ﵽƽ��
C����d��t1ʱ�̣��͵�e��t2ʱ�̣���n��N2����һ��
D�������������䣬773K�·�Ӧ��t1ʱ�̣�n��H2������ͼ��d���ֵ��
��2����֪N2��g��+3H2 ��g���T2NH3��g����H=-92.4kJ•mol-1
�ٺϳɰ���ҵ��ȡ�����д�ʩ������ƽ���ƶ�ԭ�����͵���BC����ѡ���
A�����ýϸ�ѹǿ��20MPa��50MPa�� B������500��ĸ���
C��������ý������ D�������ɵİ�Һ������ʱ����ϵ�з������
�����ݻ���Ϊ2L������������ɱ䣩�ļס������������У��ֱ����2molN2��6molH2��1molN2��3molH2������ͬ�¶ȡ�������ʹ�䷴Ӧ�����մﵽƽ���������N2ת���ʷֱ�Ϊ���������������������ƽ�ⳣ������ʽΪ$\frac{4{{��}_{��}}^{2}}{27��1-{��}_{��}��^{4}}$���ú������Ĵ���ʽ��ʾ������Ϊ���ʽ������ʱ���������������������������=������
���� ��1��A��a��b����������Ӧ���У�Ũ��Խ��Ӧ����Խ�죻
B��c�㷴Ӧ������������ʵ������ڱ仯��
C��d���e�㶼����ƽ��״̬��n��N2�����䣻
D���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ���¶�����ƽ�����淴Ӧ�ƶ���
��2������ɳ����ԭ��������ı�Ӱ��ƽ���һ����������Ũ�ȡ�ѹǿ���¶ȵȣ���ƽ������ܹ��������ָı�ķ����ƶ�����ɳ����ԭ�����õĶ���Ӧ���ڿ�����̣������������أ���ƽ���ƶ��أ���������ɳ����ԭ�����ͣ�
�ڼ���N2����ʼŨ��Ϊ$\frac{2mol}{2L}$=1mol/L��H2����ʼŨ��Ϊ$\frac{6mol}{2L}$=3mol/L��ƽ��ʱ����Ũ�ȱ仯��Ϊ����mol/L����
N2��g��+3H2��g���T2NH3��g��
��ʼŨ�ȣ�mol/L����1 3 0
�仯Ũ�ȣ�mol/L�������� 3���� 2����
ƽ��Ũ�ȣ�mol/L����1-���� 3��1-������ 2����
�ٸ���K=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}����{c}^{3}��{H}_{2}��}$����ƽ�ⳣ����
��ЧΪ��ƽ��Ļ�����ѹǿ����һ��������ѹǿƽ�������ƶ���
��� �⣺��1��A��a��b����������Ӧ���У����ŷ�Ӧ�Ľ��У���Ӧ���Ũ����С����Ӧ������С����a�������Ӧ���ʱ�b���A��ȷ��
B��c�㷴Ӧ������������ʵ������ڱ仯��û�дﵽƽ��״̬����B����
C��d���e�㶼����ƽ��״̬��n��N2�����䣬d���e��n��N2����ȣ���C����
D���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ���¶�����ƽ�����淴Ӧ�ƶ������������ʵ�������D��ȷ��
�ʴ�Ϊ��AD��
��2����A�����ڳ�ѹ������ѹǿ����ѧƽ��������ƶ��������ڰ����ĺϳɣ�������ɳ����ԭ�����ͣ���A��ѡ��
B��500��ĸ��£��������ڰ����ĺϳɣ����ǿ�����ߴ����Ĵ����ԣ���������ɳ����ԭ�����ͣ���Bѡ��
C������ý����������������ѧƽ����ƶ�����������ɳ����ԭ�����ͣ���Cѡ��
D�������ɵİ�Һ������ʱ����ϵ�з��������δ��Ӧ��N2��H2ѭ�����ϳ����У�����ʹ�û�ѧƽ�������ƶ��������ڰ��ĺϳɣ�������ɳ����ԭ�����ͣ���D��ѡ��
��ѡ��BC��
�ڼ���N2����ʼŨ��Ϊ$\frac{2mol}{2L}$=1mol/L��H2����ʼŨ��Ϊ$\frac{6mol}{2L}$=3mol/L��ƽ��ʱ����Ũ�ȱ仯��Ϊ����mol/L����
N2��g��+3H2��g���T2NH3��g��
��ʼŨ�ȣ�mol/L����1 3 0
�仯Ũ�ȣ�mol/L�������� 3���� 2����
ƽ��Ũ�ȣ�mol/L����1-���� 3��1-������ 2����
��ƽ�ⳣ��K=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}����{c}^{3}��{H}_{2}��}$=$\frac{��2{��}_{��}��^{2}}{��1-{��}_{��}����[3��1-{��}_{��}��]^{3}}$=$\frac{4{{��}_{��}}^{2}}{27��1-{��}_{��}��^{4}}$��
��ЧΪ��ƽ��Ļ�����ѹǿ����һ��������ѹǿƽ�������ƶ���N2ת�������ʦ�����������
�ʴ�Ϊ��$\frac{4{{��}_{��}}^{2}}{27��1-{��}_{��}��^{4}}$������
���� ���⿼����ۺϣ��漰���ʵ�����Ũ����ʱ��ı仯���ߡ���ѧƽ���ƶ�ԭ������ѧƽ��ļ��㣬ע�ظ߿��������Ŀ��飬��Ŀ�Ѷ��еȣ�

| A�� | NA��N2������NA��CO���ӵ�������Ϊ1��1 | |
| B�� | ˮ��Ħ����������NA��ˮ���ӵ��������֮�� | |
| C�� | �ڳ��³�ѹ��11.2LN2���еķ�����Ϊ0.5NA | |
| D�� | 1mol•L-1NaCl��Һ�С�����NA��Na+ |
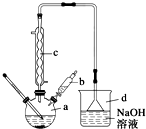 �屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�
�屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�����1����a�м���15mL����������м���ٽ�b��4.0mLҺ���������뵽a�У���ַ�Ӧ��
����2����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����3����Һ������10mLˮ��8mL 10%��NaOH��Һ��10mL ˮϴ�ӣ���Һ�ô��屽��
����4����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����˼��ôֲ�Ʒ��
| �� | �� | �屽 | |
| �ܶ�/g•cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ��ˮ�е��ܽ�� | �� | �� | �� |
��2����b�е�Һ���������뵽a�У������ܿ��ټ����ԭ���Ƿ�ֹ��Ӧ�ų�����ʹC6H6��Br2�ӷ���Ӱ����ʣ�
��3������c��������������������������Ҫ������C6H6��Br2 ���ѧʽ����
��4������4�õ��Ĵֲ�Ʒ�л��������ʱ�����֪�����屽���й������������ϱ�����Ҫ��һ���ᴿ�ֲ�Ʒ����������е�ʵ���������������
| A�� | һ������SO42- | B�� | һ������Ag+ | ||
| C�� | һ������SO42-��Ag+ | D�� | ���ܺ���SO42-��Ag+ |
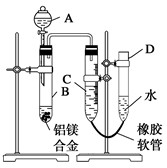 ijѧϰС������ͼ��ʾװ�òⶨ��þ�Ͻ������������������������ԭ��������
ijѧϰС������ͼ��ʾװ�òⶨ��þ�Ͻ������������������������ԭ��������
