��Ŀ����
5��W��X��Y��Z��Ԫ�����ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ���������ε����������±��е���Ϣ���ش��������⣺| W | �ڶ�������һ�ַǽ���Ԫ�أ���һ�����ܴ�������Ԫ�� |
| X | �����Ľ����������ڱ��е�������������������� |
| Y | ��̬�⻯�P������������Ӧ��ˮ�����Ϊǿ�� |
| Z | ��ԭ��������Ϊ56��������Ϊ30�ĺ��� |
��2��W��X��Y����Ԫ�صļ����Ӱ뾶�Ĵ�С��ϵ��C1-��N3-��Al3+ ���������ӷ��ű�ʾ����
��3��Y����̬�⻯��ķе���ͬ��Ԫ���γɵ���̬�⻯������� ����ߡ��͡�����XW��������ʯ���ƣ���ͬһ��Wԭ��������Xԭ�ӹ��ɵĿռ乹��Ϊ���������Σ�
��4����25�桢101 kPa�£�Z�ĵ�����������ȼ�պ�ָ���ԭ�¶Ⱥ�ѹǿ��ƽ��ÿת��1mol���ӷų�Q kJ����������Z����ȼ�յ��Ȼ�ѧ����ʽΪ3Fe��s��+2O2��g���TFe3O4��s����H=-8QkJ•mol-1 ��
��5����һ�������£�����W���⻯���ʹ������Ⱦ��֮һ��WO2ת��Ϊ�������ѭ�������ʣ���д��һ���������������ķ�Ӧ���̣�6NO2+8NH3=7N2+12H2O��
���� ���ֶ�����Ԫ��W��X��Y��Z��ԭ��������������W�ǵڶ�������һ�ַǽ���Ԫ�أ���һ�����ܴ�������Ԫ�أ���WΪ��Ԫ�أ�X�dz����Ľ����������ڱ��е�������������������ȣ���XΪAlԪ�أ�Y��̬�⻯�P������������Ӧ��ˮ�����Ϊǿ�ᣬ��YΪClԪ�أ�Z������������Ϊ56��������Ϊ30�ĺ�����ԭ������Ϊ26����ZΪFeԪ�أ��ݴ˽��
��� �⣺���ֶ�����Ԫ��W��X��Y��Z��ԭ��������������W�ǵڶ�������һ�ַǽ���Ԫ�أ���һ�����ܴ�������Ԫ�أ���WΪ��Ԫ�أ�X�dz����Ľ����������ڱ��е�������������������ȣ���XΪAlԪ�أ�Y��̬�⻯�P������������Ӧ��ˮ�����Ϊǿ�ᣬ��YΪClԪ�أ�Z������������Ϊ56��������Ϊ30�ĺ�����ԭ������Ϊ26����ZΪFeԪ�أ�
��1��WΪ��Ԫ�أ���ԭ�Ӻ���2p�ܼ��ĵ����Ų����ڰ���״̬�Ƚ��ȶ�������W�ĵ�һ�����ܴ�����ͬ�������ڵ�Ԫ�أ�ZΪFeԪ�أ�Z3+��M�������Ϊ13��
�ʴ�Ϊ����Ԫ�ص�ԭ�Ӻ���2p�ܼ��ĵ����Ų����ڰ���״̬�Ƚ��ȶ���13��
��2�����ݵ��Ӳ���Խ��뾶Խ���Ӳ�����ͬʱ���˵����Խ�뾶ԽС�������ж�W��X��Y����Ԫ�صļ����Ӱ뾶�Ĵ�С��ϵ��C1-��N3-��Al3+��
�ʴ�Ϊ��C1-��N3-��Al3+��
��3���ȵ�ͬ����F��Cl��Br��I�����⻯��ķе�����û���������Է��������йأ���ͬ����ֻ��HF���Ӽ���������HF�е���ߣ������Է���������HCl����Է���������С��������ͣ�XΪAlԪ�أ�WΪ��Ԫ�أ�XW��������ʯ���ƣ����ݽ��ʯ�Ľṹ��֪����ͬһ����ԭ����������ԭ�ӹ��ɵĿռ乹��Ϊ���������Σ�
�ʴ�Ϊ���ͣ����������Σ�
��4��ZΪFeԪ�أ���25�桢101 kPa�£�Z�ĵ�����������ȼ�պ�ָ���ԭ�¶Ⱥ�ѹǿ��ƽ��ÿת��1mol���ӷų�Q kJ������������ȼ�յķ�Ӧ��ת����8mol���ӣ���ų�������Ϊ8Q������Fe����ȼ�յ��Ȼ�ѧ����ʽΪ3Fe��s��+2O2��g���TFe3O4��s����H=-8Q kJ•mol-1 ��
�ʴ�Ϊ��3Fe��s��+2O2��g���TFe3O4��s����H=-8Q kJ•mol-1 ��
��5�����ݷ�Ӧǰ��ԭ���غ㼰����Ϣת��Ϊ�������ѭ�������ʣ���ʽΪ6NO2+8NH3=7N2+12H2O���ʴ�Ϊ��6NO2+8NH3=7N2+12H2O��
���� ���⿼��ṹ����λ�ù�ϵ���е�Ƚϡ�Ԫ�ػ��������ʵȣ��Ѷ��еȣ��ƶ�Ԫ���ǽ���ؼ���

 ͼ�С�-����ʾ���������ʼ���һ�������¿��Է�Ӧ����-������ʾ����һ�������¿���ת��Ϊ�ң���������ѡ���У�����ͼʾҪ����ǣ�������
ͼ�С�-����ʾ���������ʼ���һ�������¿��Է�Ӧ����-������ʾ����һ�������¿���ת��Ϊ�ң���������ѡ���У�����ͼʾҪ����ǣ�������| �� | �� | �� | �� | |
| A | H2SO4 | Na2SO4 | NaOH | NaCl |
| B | KCl | K2CO3 | KOH | HCl |
| C | O2 | CO | CuO | C |
| D | Fe | CuCl2 | Zn | HCl |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ������Һ | B�� | ���Ƶ�������ͭ | C�� | ��ˮ | D�� | ����������Һ |
| A�� | ���������Һ��ͨ������CO2���塡SiO32-+CO2+H2O�TH2SiO3��+CO32- | |
| B�� | ��CuƬ����FeCl3��Һ�С�Cu+Fe3+�TFe2++Cu2+ | |
| C�� | ���ռ���Һ�еμ�����Al2��SO4��3��Һ��Al3++4OH-�T[Al��OH��4]- | |
| D�� | ��Ag˿����NaNO3��H2SO4�Ļ��Һ�С�3Ag+4H++NO3-�T3Ag++NO��+2H2O |
| A�� | ͬһ����Ԫ�أ�ԭ�Ӱ뾶Խ���䵥�ʵ��۵㲻һ��Խ�� | |
| B�� | ȫ���ɷǽ���Ԫ����ɵĻ�������һ��ֻ�й��ۼ� | |
| C�� | ����пƬ��ͭƬ��ϡ������ɵ�ԭ����У�������ͭƬ�����·����пƬ | |
| D�� | �Ȼ��ƺ��Ȼ���ֱ��ܽ���ˮ�����˷������Ӽ����������ͬ������ |
| A�� | NaHA��Һһ�������� | B�� | c��H+��•c��OH-��=1��10-14x | ||
| C�� | c��Na+��=c��K+�� | D�� | c��Na+��+c��K+��=0.05 mol/L |
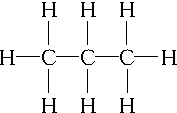 ��C3H6��ͬ���칹��ΪCH2�TCH-CH3��
��C3H6��ͬ���칹��ΪCH2�TCH-CH3��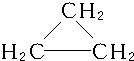 ��
��