��Ŀ����
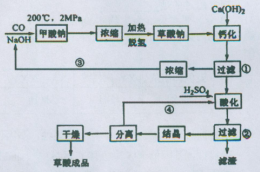
�������ƣ�NaClO2����Ҫ�����ġ���ֽҵ��Ư����Ҳ����ʳƷ������ˮ�����ȣ��������������ֽ⡣�������Ƶ�Ϊԭ���Ʊ��������ƵĹ����������£�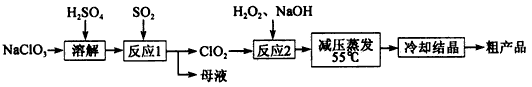
��1����ߡ���Ӧl����Ӧ���ʵĴ�ʩ��______________��д��һ�����ɣ���
��2������Ӧ2������������________���÷�Ӧ�Ļ�ѧ����ʽΪ__________��
��3����ȡ����ѹ�����������á���ѹ��������ԭ����__________________��
��4���� ��ĸҺ���пɻ��յ���Ҫ������__________________________��
��5������ȴ�ᾧ����_____________����������ƣ����ɻ�ôֲ�Ʒ��
��1�������¶ȣ���������ҺŨ�ȵȡ�
��2��ClO2 H2O2+2ClO2+2NaOH=2NaClO2+2H2O+O2
��3����ѹ�����¶ȹ��ߣ������������ֽ⣻
��4��Na2SO4
��5������
����

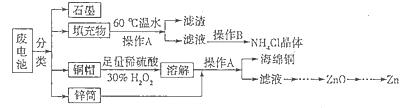
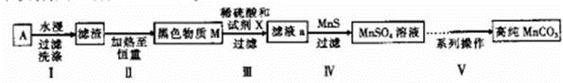
�����к��ḻ�ĵ�Ԫ�أ��Ӻ����з������ɰ����²�����У��ٵμ�ϡ�����ữ��������H2O2����������Ϊ3%�� �ڽ��������ճɻҺ��ˮ���� �ۼ�CCl4�� ���÷�Һ©����Һ ����С����ˡ������IJ���˳����
| A���٢ڢۢܢ� | B���٢ۢݢڢ� | C���ڢݢ٢ۢ� | D���ڢ٢ۢݢ� |
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ� Fe2����Mg2����Al3����Ba2����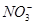 ��
��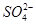 ��Cl����I����
��Cl����I���� ��ȡ����Һ����ʵ�飺
��ȡ����Һ����ʵ�飺
| ʵ�鲽�� | ʵ������ |
| (1)ȡ��������Һ���Ӽ�����ɫʯ����Һ | ��Һ��� |
| (2)ȡ��������Һ���ȣ���CuƬ��ŨH2SO4������ | ����ɫ���������������������ɺ���ɫ |
| (3)ȡ��������Һ����BaCl2��Һ | �а�ɫ���� |
| (4)ȡ(3)���ϲ���Һ����AgNO3��Һ | �а�ɫ�������Ҳ�����ϡHNO3 |
| (5)ȡ��������Һ����NaOH��Һ | �а�ɫ������NaOH����ʱ���������ܽ� |
�ɴ��жϣ�
(1)��Һ�п϶������ڵ������� ����Һ�п϶����ڵ������� ��
(2)�����ʵ����֤���п��ܴ��ڵ������ӵķ���(д��������������) ��

 ���Ҵ���
���Ҵ���