��Ŀ����
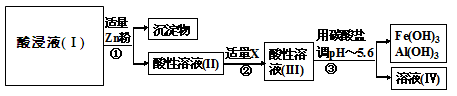
��14�֣��Ͼɼ���п�̸ɵ���ڲ��ĺ�ɫ����A��Ҫ����MnO2��NH4CI��ZnCI2������������FeCI2��̿�ۣ���A�Ʊ��ߴ�MnCO3��������ͼ���¡�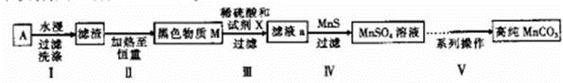
��1������п�̸ɵ�صĸ���������_________(�ѧʽ)��
��2���ڢ�����Ŀ����________________________��
��3���ڢ��������Ƕ���Һa������ȳ��ӣ���ȥZn2+�����ӷ���ʽΪ____________________��
���� (��֪��Ksp(MnS)=2.5��10-13��Ksp(ZnS)=1.6��10-24)
��4��Ϊѡ���Լ�X������ͬ�����£��ֱ���5 g��ɫ����M�����Ʊ�MnSO3��ʵ�飬�õ��������ұ���
�����Լ�x�����ѡ����_________��
���ڵڢ�����ѡ��������Լ�X��M����Ҫ�ɷַ�Ӧ�Ļ�ѧ����ʽΪ_________��
��5����֪��MnCO3������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻Mn(OH)2��ʼ����ʱpHΪ7.7���벹��������²�����
�ڢ���ϵ�в����ɰ�һ�����̽��У��벹����ɲ��������ڢ���ϵ�в����пɹ�ѡ�õ��Լ��� ���Ҵ���
���Ҵ���
����1��___________________������2�����ˣ�������ˮϴ��2��3��
����3�������Һ������SO42-�ѳ��ɾ���������4��___________________��
����5�����º�ɡ�
��6������1���ܷ�����Ӧ�����ӷ���ʽ
��14�֣�ÿ��2�֣�
��1��Zn
��2����ȥ̼��
��3��Zn2++MnS=ZnS+Mn2+
��4����30%�������� ��H2O2+MnO2+H2SO4=MnSO4+2H2O+O2��
��5������NaHCO3������pH<7.7�� ��������ˮ�Ҵ�ϴ��2��3��
��6��Mn2++2 HCO3-= MnCO3��+H2O+CO2��
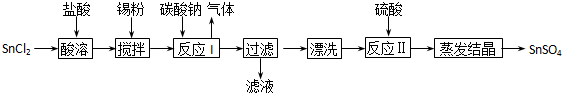
���������������1�� ����п�̸ɵ���У�п���̻��ã����Ը���������Zn��
��2����I������ˮ��������ˮ��NH4CI��ZnCI2�ɳ�ȥ����̼�۲�����ˮ�����Եڢ�����Ŀ���dz�ȥ̼�ۣ�
��3���ڢ��������м���MnSĿ�������ó�����ת����ȥZn2+��ʹMnSת��Ϊ�����ܵ�ZnS�����ӷ���ʽΪZn2++MnS=ZnS+Mn2+
��4���ٸ��ݱ�������ѡ��30%�Ĺ�������õ���MnSO4��������࣬����ѡ��30%�Ĺ������⣻
�ڸ��ݱ���֪�����������ǰѶ�������ת��Ϊ�����̣�����������Ϊ��������ѧ����ʽΪH2O2+MnO2+H2SO4=MnSO4+2H2O+O2��
��5�����ղ�����̼���̣����Ե�һ��Ӧ����NaHCO3������pHֵʹ֮С��7.7����ֹ�����������ɣ�MnCO3��ʪʱ�ױ���������������ˮϴ���������������ˮ�Ҵ�ϴ��2��3���Գ�ȥ̼���̱����ˮ�֣�
��6������1Ŀ��������̼���̵ij��������Կ��ܷ�����Ӧ�����ӷ���ʽMn2++2 HCO3-= MnCO3��+H2O+CO2��
���㣺����ԭ����е缫���жϣ�����Ӧ����Ŀ����Ϣ����������ѧ����ʽ���жϼ���д

 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
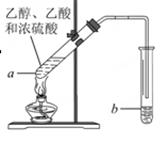
��ͨ����ͬ����ϰ��ϵ�д�ʵ������Ũ���ᡢŨ����Ļ�����뱽��Ӧ��ȡ���������õ��ֲ�Ʒ��Ҫѡ�����¼��������Դֲ�Ʒ���о��ƣ������� ��ˮϴ ���ø�������и��� ����10%��NaOH��Һϴ�ӡ���ȷ�IJ���������
| A���٢ڢۢܢ� | B���ڢܢڢۢ� | C���ܢڢۢ٢� | D���ۢܢ٢ڢ� |
�����й�ʵ��ԭ���������ͽ��۶���ȷ����
| A����������������Һ�����ȣ�������������ʹʪ���ɫʯ����ֽ������һ����NH4+ |
| B��ȡ����X��Һ����������������ˮ���ټӼ���KSCN��Һ����Һ��죬˵��X��Һ��һ������Fe2�� |
| C����ij��Һ�еμ�BaCl2��Һ�����а�ɫ�������ٵμ�����ϡHNO3�����������ܽ⣬��˵��ԭ��Һ��һ����SO42- |
| D��ij��ɫ��Һ�ýྻ��˿պȡ��Һ������ɫ��Ӧ������ʻ�ɫ����ԭ��Һ����Na+��K+ |
����þ��ҽҩ����������ҵӦ�ù㷺������þ��ԭ�Ƚ��Ʊ��ߴ�����þ��һ���µ�̽��������þ��(��Ҫ�ɷ�ΪMgCO3��������FeCO3 )Ϊԭ���Ʊ��ߴ�����þ��ʵ���������£�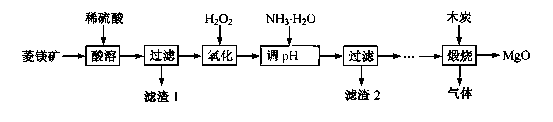
�����������������pH
| | Mg(OH)2 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����ʱ | 9.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 12.4 | 8.3 | 2.8 |
��1��MgCO3��ϡ���ᷴӦ�����ӷ���ʽΪ ��
��2���Ӱ�ˮ������Һ��PH��ΧΪ ��
��3������2 �ijɷ��� (�ѧʽ)��
��4�����չ��̴������·�Ӧ��
2MgSO4+C
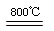 2MgO+2SO2��+CO2��
2MgO+2SO2��+CO2��MgSO4+C
 MgO+SO2��+CO��
MgO+SO2��+CO�� MgSO4+3C
 MgO+S��+3CO��
MgO+S��+3CO��������ͼװ�ö����ղ�����������зֲ����ջ��ռ���

��D���ռ��������� (�ѧʽ)��
��B��ʢ�ŵ���Һ�� (����ĸ)��
a��NaOH ��Һ b��Na2CO3��Һ c��ϡ���� d��KMnO4��Һ
��A�еõ��ĵ���ɫ�������ȵ�NaOH��Һ��Ӧ��������Ԫ�����̬Ϊ+4��д���÷�Ӧ�����ӷ���ʽ�� ��