��Ŀ����
����Ŀ����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����NH4����Cl-��Mg2����Ba2����CO32-��SO42-����ȡ���ݸ�100mL��Һ��������ʵ�飺
��һ�ݼ���AgNO3��Һ�г����������ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ���0.08mol���塣�����ݼ�����BaCl2��Һ�õ��������12.54g������������ϴ�ӡ������������Ϊ4.66g��
��������ʵ�飬�ش��������⣺
��1���ɵ�һ�ݽ��е�ʵ���ƶϸû�����Ƿ�һ������Cl-��__��
��2���ɵڶ��ݽ��е�ʵ���֪�������Ӧ����__�������ʵ���Ũ��Ϊ__��
��3���ɵ����ݽ��е�ʵ���֪12.54g�����ijɷּ����ʵ�����__��
��4���ۺ�����ʵ�飬����Ϊ���½�����ȷ����__��
A.�û��Һ��һ������K����NH4��![]() ��CO32-��SO42-�����ܺ�Cl-����n(K��)��0.04mol
��CO32-��SO42-�����ܺ�Cl-����n(K��)��0.04mol
B.�û��Һ��һ������NH4����CO32-��SO42-�����ܺ�K����Cl-
C.�û��Һ��һ������NH4����CO32-��SO42-�����ܺ�Mg2����K����Cl-
D.�û��Һ��һ������NH4����SO42-�����ܺ�Mg2����K����Cl-
���𰸡���һ�� NH4+ 0.8mol/L 0.04mol BaCO3��0.02mol BaSO4 A
��������
�������Ӽ���ķ�����ʵ��������Һ�ʵ����Է�����Һ�д��ڵ����ӡ�
��1������̼������ӡ���������Ӷ��ܹ��������ӷ�Ӧ����̼������������������������ȷ��ԭ��Һ���Ƿ���������ӣ��ʴ�Ϊ����һ������Ϊ̼���������������dz�����
��2��������NaOH��Һ���Ⱥ��ռ���0.08mol���壬������Ϊ������˵����Һ�� һ����������ӣ�����ӵ����ʵ���Ũ��Ϊ��c��NH4+��=![]() =0.8mol/L���ʴ�Ϊ��NH4+��0.8mol/L��
=0.8mol/L���ʴ�Ϊ��NH4+��0.8mol/L��
��3��������BaCl2��Һ�õ��������12.54g������������ϴ�ӡ������������Ϊ4.66g��˵������Ϊ���ᱵ��̼�ᱵ�Ļ�������4.66gΪ���ᱵ������n��BaSO4��=n��SO42-��=![]() =0.02mol��̼�ᱵ����������Ϊ��12.54g-4.66g=7.88g������n��BaCO3��=n��CO32-��=
=0.02mol��̼�ᱵ����������Ϊ��12.54g-4.66g=7.88g������n��BaCO3��=n��CO32-��=![]() =0.04mol���ʴ�Ϊ��0.04mol BaCO3��0.02mol BaSO4��
=0.04mol���ʴ�Ϊ��0.04mol BaCO3��0.02mol BaSO4��
��4���������Ϸ�����֪���ٸ��ݵ���غ㣬�����Ϊ��n��+��=n��NH4+��=0.08mol��n��-��=2n��CO32-��+2n��SO42-��=0.12mol����һ����K+������0.04mol������Һ��һ�����ڣ�K+��NH4+��CO32-��SO42-�����ܺ���Cl-�������������ӣ������ӵ����ʵ�������0.04mol���������������ӣ������ӵ����ʵ���Ϊ0.04mol������A��ȷ���ʴ𰸰�Ϊ��A��

����Ŀ����̼���ʵļ�ֵ��ת��������������̼���Ϳɳ����Է�չ��������Ҫ���о���ֵ����ش��������⣺
(1)��֪CO�����л�ѧ��ΪC��O����صĻ�ѧ�������������£�
��ѧ�� | H��O | C��O | C=O | H��H |
E/(kJ��mol1) | 463 | 1075 | 803 | 436 |
CO(g)+H2O(g)CO2(g)+H2(g) ��H=__kJ��mol1��
�������������COƽ��ת���ʵĴ�ʩ��__(����)��
a.����ѹǿ b.�����¶� c.���ԭ������H2O�ı��� d.ʹ�ø�Ч����
(2)�ö��Ե缫���KOH��Һ���ɽ������е�CO2ת��Ϊ�����(HCOO)��Ȼ���һ�������Ƶ���Ҫ�л�����ԭ�ϼ��ᡣCO2������Ӧ�ĵ缫��ӦʽΪ__������������ת��1mol���ӣ�����������������(��״��)Ϊ__L��
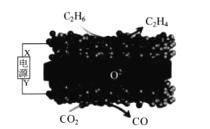
(3)�ұ���������ȡ����ϩ�ķ�ӦΪ��
![]() (g)+CO2(g)
(g)+CO2(g)![]() (g)+CO(g)+H2O(g)���䷴Ӧ�������£�
(g)+CO(g)+H2O(g)���䷴Ӧ�������£�

��һ���¶��£�������ܱ������г���2mol�ұ���2molCO2����ʼѹǿΪp0��ƽ��ʱ���������������ʵ���Ϊ5mol���ұ���ת����Ϊ__����ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp=__��[�����ѹ(p��)=������ѹ(p��)�������������]
���ұ�ƽ��ת������p(CO2)�Ĺ�ϵ��ͼ��ʾ��������ұ�ƽ��ת��������p(CO2)�仯���仯��ԭ��__��