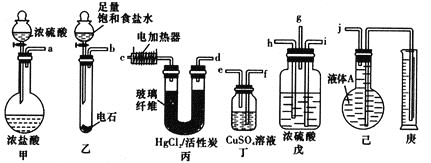
��Ŀ����
����Ŀ��ij��ӦaA(g)+bB(g)![]() cC(g) H<0�������Ϊ2L���ܱ������з�Ӧ����I��II��III��ͬ����ϵ�иı�ijһ��������ϵ�и����ʵ���(mol)��ʱ��仯��������ͼ��ʾ������˵������ȷ���ǣ�
cC(g) H<0�������Ϊ2L���ܱ������з�Ӧ����I��II��III��ͬ����ϵ�иı�ijһ��������ϵ�и����ʵ���(mol)��ʱ��仯��������ͼ��ʾ������˵������ȷ���ǣ�
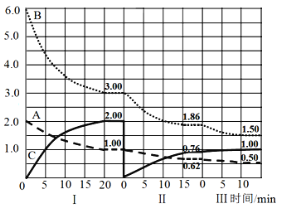
A.�÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ��A+3B![]() 2C
2C
B.������A��ʾ��I��20������ƽ����Ӧ����Ϊ��v(A)=0.025mol��L-1��min-1
C.�����η�Ӧ��ƽ�ⳣ����ϵΪ��KI<KII<KIII
D.�����η�Ӧ������Ϊ��v(B)I>v(B)II>v(B)III��������ת����AI%>AII%>AIII%
���𰸡�C
��������
��ͼ��֪���ṩ6.0molB��2.0molA���ڵ�I�η�����Ӧ����ƽ�⣻�ڵ�II�ν�C�����ʵ�����Ϊ0(��С����������ʵ���)����ʱA��B�����ʵ������䣬ƽ�������ƶ����ڵ����Σ���ʼʱ��A��B��C�����ʵ��������䣬��ƽ���ƶ���A��B�����ʵ�����С��C�����ʵ���������ƽ�������ƶ�����ı������Ϊ���¡�
A���ڵ�I�Σ�A��B�����ʵ����ֱ����1mol��3mol��C�����ʵ�������2mol����÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ��A+3B![]() 2C��A��ȷ��
2C��A��ȷ��
B����I��20������ƽ����Ӧ����Ϊ��v(A)=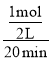 =0.025mol��L-1��min-1��B��ȷ��
=0.025mol��L-1��min-1��B��ȷ��
C��I��II�ε��¶���ͬ��KI=KII����III�Σ�����ƽ�������ƶ���ƽ�ⳣ������KII<KIII�����Է�Ӧ��ƽ�ⳣ����ϵΪ��KI=KII<KIII��C����ȷ��
D��������B�����ʵ����ı仯���ֱ�Ϊ3mol��1.14mol��0.36mol����Ӧ������Ϊ��v(B)I>v(B)II>v(B)III��������A��ת���ʷֱ�Ϊ50%��38%��19%����ת����AI%>AII%>AIII%��D��ȷ��
��ѡC��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����0.1500mol/L��HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ��ʵ���������±���ʾ��
ʵ���� | ����NaOH��Һ�����/mL | HCl��Һ�����/mL |
1 | 25.00 | 24.41 |
2 | 25.00 | 24.39 |
3 | 25.00 | 25.60 |
�ش��������⣺
��1��ʵ���У���Ҫ��ϴ�������ǣ�________________________����д�������ƣ���
��2��ȡ����ҺNaOH��Һ25.00ml ����ƿ�У�ʹ�÷�̪��ָʾ�����ζ��յ���ж�������______________
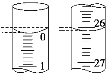
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ��������������Һ�����Ϊ________mL��
��4�����в����л�ʹ������ƫ�ߵ���_________________��ƫ�͵���_________________
����ʽ�ζ���©Һ���ڵζ�ǰ��ʽ�ζ��ܼ��첿�������ݣ��ζ����������ݱ�С���۵ζ������У�����ƿʱ����С�Ľ���Һ�������ܵζ������У���ƿ�ڼ���������ˮ; ���ü�����ָʾ�����еζ�ʱ����Һ�ɳ�ɫ���ɫʱֹͣ�ζ������ü�����ָʾ������Һ�ɻ�ɫ���ɫ��5 s���ֱ�Ϊ��ɫ���߶���ʽ�ζ��ܶ���ʱ���ζ�ǰ���Ӷ���
��5��δ֪Ũ�ȵ�NaOH��Һ�����ʵ���Ũ��Ϊ_____________mol/L��