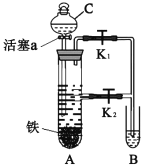
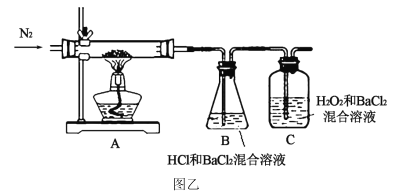
��Ŀ����
����Ŀ��M�ǽ����������̵�ĸ߷����л��������ṹ��ʽΪ ����ʯ���ѽ����ϳ�M��·������
����ʯ���ѽ����ϳ�M��·������
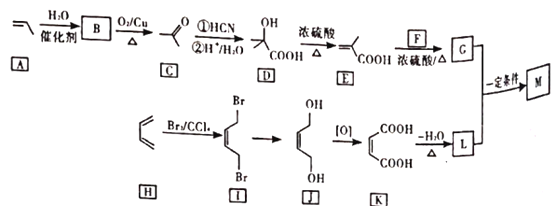
�ش��������⣺
(1)B�Ļ�ѧ����Ϊ___________��
(2)F�ķ���ʽΪ___________��
(3)G�й����ŵ�������___________��G��L��һ������������M�ķ�Ӧ������___________��
(4)I��J�ķ�Ӧ����ʽΪ___________��
(5)д��C��ͬ���칹��Ľṹ��ʽ(�˴Ź�������Ϊ����壬�������Ϊ3�U2�U1��______��
(6)����ɱ���ϩΪ��ʼԭ���Ʊ�![]() �ĺϳ�·��___________(���Լ���ѡ)��
�ĺϳ�·��___________(���Լ���ѡ)��
���𰸡�2-���� C16H34O ̼̼˫�������� �Ӿ۷�Ӧ ![]() +2NaOH
+2NaOH![]()
![]() +2NaBr CH3CH2CHO��
+2NaBr CH3CH2CHO��![]()
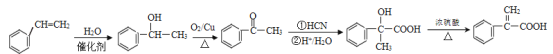
��������
(1)����ͼʾ��֪A��CH3-CH=CH2����������H2O��һ�������·����ӳɷ�Ӧ����![]() ������Ϊ2-�������������
������Ϊ2-�������������
(2)M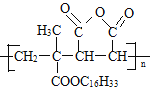 ����G(
����G(![]() )��L(
)��L(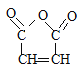 )�����Ӿ۷�Ӧ��Ӧ�����ĸ߾��
)�����Ӿ۷�Ӧ��Ӧ�����ĸ߾��![]() ����E(
����E(![]() )��F����������Ӧ�γɵģ�����F�Ǵ���F�ķ���ʽΪC16H34O��
)��F����������Ӧ�γɵģ�����F�Ǵ���F�ķ���ʽΪC16H34O��
(3)G�Ľṹ��ʽ��![]() �����й����ŵ�������̼̼˫����������G��L��һ������������M�ķ�Ӧ�����ǼӾ۷�Ӧ��
�����й����ŵ�������̼̼˫����������G��L��һ������������M�ķ�Ӧ�����ǼӾ۷�Ӧ��
(4)I��![]() ����������NaOH��ˮ��Һ�ڼ��������·���ˮ�ⷴӦ(��ȡ����Ӧ)����
����������NaOH��ˮ��Һ�ڼ��������·���ˮ�ⷴӦ(��ȡ����Ӧ)����![]() ������I��J�ķ�Ӧ����ʽΪ
������I��J�ķ�Ӧ����ʽΪ![]() +2NaOH
+2NaOH![]()
![]() +2NaBr��
+2NaBr��
(5)��C�ṹ��ʽ��֪C�ķ���ʽ��C3H6O������ͬ���칹���У��˴Ź�������Ϊ����壬�������Ϊ3�U2�U1�Ľṹ��ʽ��CH3CH2CHO ��![]() ��
��
(6) ![]() �к��й�����Ϊ̼̼˫�����Ȼ����Աȱ���ϩ��
�к��й�����Ϊ̼̼˫�����Ȼ����Աȱ���ϩ��![]() �Ľṹ��ʽ����������һ��̼ԭ�ӣ�ģ��������A��B��C��D��ʵ��̼�ɹǼܵĹ����������Ȼ��������ȥ���ǻ����ɣ��ɱ���ϩΪ��ʼԭ���Ʊ�
�Ľṹ��ʽ����������һ��̼ԭ�ӣ�ģ��������A��B��C��D��ʵ��̼�ɹǼܵĹ����������Ȼ��������ȥ���ǻ����ɣ��ɱ���ϩΪ��ʼԭ���Ʊ�![]() �ĺϳ�·��Ϊ
�ĺϳ�·��Ϊ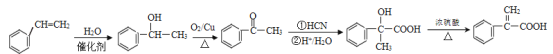 ��
��