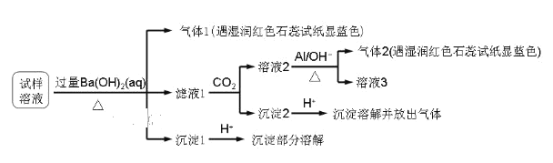
��Ŀ����
����Ŀ����������(ClO2)�Ǹ�Ч��ȫ������ˮ���������õ�Խ��Խ�㷺��Ӧ�á��ش��������⣺
I.ʵ���Һϳ�ClO2���������˫��ˮ��ԭ������(NaClO3)��
��1�������NaClO3��Ӧʱ��ClO2��Cl2���ɣ���д��Ӧ�����ӷ���ʽ���������ת�Ƶķ������Ŀ______________________________________������Ӧ��ת��0.2mol����ʱ���μӷ�Ӧ�Ļ�ԭ�������ʵ���Ϊ________________________________��
��2��ʹ��˫��ˮ����ԭ�����ŵ���_________________________________________��
II.��pH��2.0ʱ��NaClO2�ܱ�I ��ȫ��ԭ��Cl�������ӷ�Ӧ����ʽΪClO2+4H++4I��2I2+Cl+2H2O�����ش��������⣺
��Һ��Na2S2O3����I2��Ӧ����NaI��Na2S4O6��2Na2S2O3+I2��2NaI+Na2S4O6�������ⶨ��Ʒ��NaClO2�ĺ������ֽ������²�����
������ | ��ȡ��ƷWg�����Һ������ƿ����������pH��2.0 |
������ | ����ƿ�м�������KI���壬��ֽ��裬����������ָʾ�� |
������ | ��c mol/L��Na2S2O3��Һ�ζ�������Ӧ |
��1������������ָʾ����������_______________���ζ��յ�ʱ��Һ����ɫ�仯Ϊ_________��
��2���������ζ�������ƽ������VmL Na2S2O3����Һ�����Ʒ��NaClO2����������Ϊ__________(�ú�W��c��V�Ĵ���ʽ��ʾ)��
��3��ClO2��Cl2���ܽ���Ʒ�ˮ�е�CN ����Ϊ�������ʣ���������ԭΪCl����������ͬ��CN�ĵ�Ʒ�ˮ������Cl2�����ʵ�����ClO2��___����
���𰸡�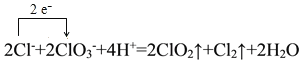 0.2mol ʹ��˫��ˮ����ԭ��ʱ��������Ϊ�������Ի�������Ⱦ ������Һ ��Һ����ɫ�����ɫ
0.2mol ʹ��˫��ˮ����ԭ��ʱ��������Ϊ�������Ի�������Ⱦ ������Һ ��Һ����ɫ�����ɫ ![]() 2.5
2.5
��������
I.(1)�����NaClO3��Ӧʱ��ClO2��Cl2���ɣ���Ӧ��HClΪ��ԭ����������������������Ԫ�صĻ��ϼ���-1�����ߵ�0�ۣ�NaClO3����ԭ��ClO2����Ԫ�ػ��ϼ۴�+5�۱�Ϊ+4�ۣ��ݴ���д���ӷ���ʽ�ͼ��㻹ԭ�������ʵ�����
(2)˫��ˮ����ԭ��������Ϊ������������ɫ��ԭ������Ⱦ��
II.(1)������з�����Ӧ��������Һ��ClO2-�ܱ�I-��ȫ��ԭ��Cl-�������ӱ�����Ϊ�ⵥ�ʣ���Һ��Na2S2O3����I2��Ӧ��2Na2S2O3+I2=2NaI+Na2S4O6�������õ�����Һ��ָʾ�����жϷ�Ӧ�յ㣻
(2)����ClO2-+4H++4I-=2I2+Cl-+2H2O��2Na2S2O3+I2=2NaI+Na2S4O6��ȷ����Ӧ�Ķ�����ϵ���м��㣻
(3)ÿĦ��Cl2�õ�2mol���ӣ���ÿĦ��ClO2�õ�5mol���ӣ���Ϊ2.5����
I.(1)�����NaClO3��Ӧʱ��ClO2��Cl2���ɣ���Ӧ�����ӷ���ʽΪ 2ClO3-+4H++2Cl-=2ClO2��+Cl2��+2H2O���������ת�Ƶķ������ĿΪ��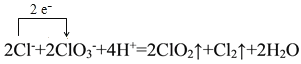 ����Ӧ��HClΪ��ԭ����������������������Ԫ�صĻ��ϼ���-1�����ߵ�0�ۣ���ת��0.2mol����ʱ���μӷ�Ӧ�Ļ�ԭ�������ʵ���ΪΪ0.2mol��
����Ӧ��HClΪ��ԭ����������������������Ԫ�صĻ��ϼ���-1�����ߵ�0�ۣ���ת��0.2mol����ʱ���μӷ�Ӧ�Ļ�ԭ�������ʵ���ΪΪ0.2mol��
(2)˫��ˮ����ԭ��ʱ��������Ϊ�������Ի�������Ⱦ��
II.(1)������з�����Ӧ��������Һ��ClO2-�ܱ�I-��ȫ��ԭ��Cl-�������ӱ�����Ϊ�ⵥ�ʣ����ӷ���ʽΪ��ClO2-+4H++4I-=2I2+Cl-+2H2O����Һ��Na2S2O3����I2��Ӧ��2Na2S2O3+I2=2NaI+Na2S4O6�������õ�����Һ��ָʾ�����ζ��յ�ʱ��Һ����ɫ�仯Ϊ����Һ����ɫ��Ϊ��ɫ��
(2)�ζ������з����ķ�ӦΪ��ClO2-+4H++4I-=2I2+Cl-+2H2O��2Na2S2O3+I2=2NaI+Na2S4O6����Ӧ�Ķ�����ϵΪNaClO2��2I2��4Na2S2O3����n��NaClO2��=![]() mol����Ʒ��NaClO2����������=
mol����Ʒ��NaClO2����������= ��100%=
��100%=![]() ��
��
(3)ÿĦ��Cl2�õ�2mol���ӣ���ÿĦ��ClO2�õ�5mol���ӣ�������Cl2�����ʵ�����ClO2�ģ�![]() =2.5����
=2.5����