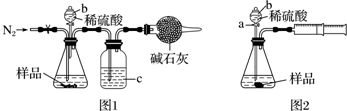
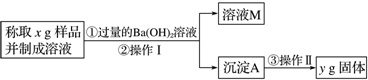
��Ŀ����
14��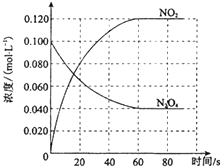 ���ݻ�Ϊ1.00L�������У�ͨ��һ������N2O4��������ӦN2O4��g���T2NO2��g����H�����¶����ߣ�����������ɫ���
���ݻ�Ϊ1.00L�������У�ͨ��һ������N2O4��������ӦN2O4��g���T2NO2��g����H�����¶����ߣ�����������ɫ����ش��������⣺
��1����Ӧ�ġ�H��0���������������100��ʱ����ϵ�и�����Ũ����ʱ��仯����ͼ��ʾ����0��60sʱ�Σ�ƽ����Ӧ����v��NO2��Ϊ0.0020mol?L-1?s-1����Ӧ��ƽ�ⳣ��KΪ0.36��
��2��100��ʱ�ﵽƽ��ı䷴Ӧ�¶�ΪT��c��N2O4�� ��0.0020mol?L-1?s-1��ƽ�����ʽ��ͣ���10s�ִﵽƽ�⣮��T��100�棨�������������
��3��100��ʱ�ﵽƽ�������������ͨ��0.1N2O4mol����ѧƽ�����ƣ�����ơ��������ơ����ƶ��������ﵽ��ƽ��ʱN2O4��Ũ�ȣ�0.04mol/L��
���� ��1�����¶����ߣ�����������ɫ���˵�������¶�ƽ�������ƶ���������ӦΪ���ȷ�Ӧ������v=$\frac{��c}{��t}$����v��NO2����
��ͼ��֪��ƽ��ʱc��NO2��=0.120mol•L-1��c��N2O4��=0.040mol•L-1������ƽ�ⳣ��K=$\frac{{c}^{2}��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$���㣻
��2���ı��¶Ⱥ�N2O4��Ũ�Ƚ��ͣ�ƽ��������Ӧ�����ƶ�����������Ӧ���ȷ�Ӧ�������¶�ƽ�������ƶ���
��3��100��ʱ�ﵽƽ�������������ͨ��0.1N2O4mol������N2O4��Ũ��������ƽ�������ƶ���NO2Ũ������һ���¶���ƽ�ⳣ�����䣬�ʴﵽ��ƽ��ʱN2O4��Ũ������
��� �⣺��1�����¶ȵ����ߣ�����������ɫ���˵�������¶�ƽ�������ƶ���������ӦΪ���ȷ�Ӧ������H��0��
0��60sʱ�Σ�NO2Ũ�ȱ仯Ϊ0.12mol/L����v��NO2��=$\frac{0.12mol/L}{60s}$=0.0020mol•L-1•s-1��
��ͼ��֪��ƽ��ʱc��NO2��=0.120mol•L-1��c��N2O4��=0.040mol•L-1����ƽ�ⳣ��K=$\frac{{c}^{2}��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$=$\frac{0.1{2}^{2}}{0.04}$=0.36��
�ʴ�Ϊ������0.0020mol•L-1•s-1��0.36��
��2���ı��¶Ⱥ�N2O4��Ũ�Ƚ��ͣ�ƽ��������Ӧ�����ƶ�����������Ӧ���ȷ�Ӧ�������¶�ƽ�������ƶ�����T��100�棬
�ʴ�Ϊ������
��3��100��ʱ�ﵽƽ�������������ͨ��0.1N2O4mol������N2O4��Ũ��������ƽ�������ƶ���NO2Ũ������һ���¶���ƽ�ⳣ�����䣬�ʴﵽ��ƽ��ʱN2O4��Ũ�ȣ�0.04mol/L��
�ʴ�Ϊ�����ƣ�����
���� ���⿼�黯ѧƽ�������Ӱ�����ء�ƽ�ⳣ����Ӱ�����ء���ѧ��Ӧ���ʵļ���ȣ��ѶȲ��������ڻ���֪ʶ�Ĺ��̣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| ѡ�� | ���� | ���� | ���� |
| A | ��AgCl����Һ�еμ�NaI��Һ | ���ֻ�ɫ���� | Ksp��AgCl����Ksp��AgI�� |
| B | �����½�AlƬ����Ũ������ | �����Ա仯 | Al��Ũ�����Ӧ |
| C | ��AgNO3��Һ�еμӹ�����ˮ | ��Һ���� | Ag+��NH3•H2O�ܴ������� |
| D | ��ˮ����ͨ�����ȵ����� | ��ĩ��� | ����ˮ�ڸ����·�����Ӧ |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |
| A�� | 0.15mol/L��H2SO4��Һ | B�� | 0.2mol/L���� | ||
| C�� | 0.2mol/L��HCl��Һ | D�� | 0.2mol/L��NaOH��Һ |
| A�� | ��ϡ��ˮ������Һ�еı��� | |
| B�� | ��NaOH��Һ����ֲ����������� | |
| C�� | ��ȼ�ա�����ζ�ķ�����������˿�Ͳ�˿ | |
| D�� | �����Ƶ�Cu��OH��2����Һ����HCHO��HCOOH |
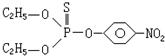 ����˵����ȷ���ǣ�������
����˵����ȷ���ǣ�������| A�� | ���л������ڷ����� | B�� | ���л���������ˮ | ||
| C�� | ���л��ﲻ�ܷ����ӳɷ�Ӧ | D�� | ���л����ܷ���ȡ����Ӧ |
| A�� | Na2CO3��NaHCO3�ȶ��ö� | |
| B�� | NaHCO3��Na2CO3������ˮ | |
| C�� | �����ʵ�����ͬ���մ��С�մ�ɵõ���ͬ������CO2 | |
| D�� | ʯ��ˮ���ܺ�Na2CO3��Ӧ��������NaHCO3��Ӧ���ҷ�Ӧԭ����ͬ |
| A�� | 1.0L1.0mo1•L-1��NaNO3ˮ��Һ�к��е���ԭ����Ϊ3NA | |
| B�� | 0.1molCu��������Ũ���ᷴӦ����SO2�����Ϊ2.24L | |
| C�� | 25��ʱpH=13��Ba��OH��2��Һ�к���OHһ����ĿΪ0.1NA | |
| D�� | ��ӦCl2+2NaOH=NaCl+NaClO+H2O�У�ÿ����1molCl2ת�Ƶ�����Ϊ NA |