��Ŀ����
19��ʵ����Ҫ0.1mol•L-1�� NaOH��Һ450mL��������Һ�����е�����ش��������⣺��1��ʵ���г���������ƽ���ձ�������ƿ���Ҫ�������������в���������ͷ�ιܣ�
��2������ƿ��������������е�ace������ţ�
a���¶�b��Ũ��c������d��ѹǿe���̶���
��3�����ݼ����֪������NaOH������Ϊ2.0g��
��4������һ�����ʵ���Ũ����Һ��ʵ���У�����������²�����
A������ʱ�������������B����NaOH����ֽ���ϳ���
C��������ƿת��ʱ��������Һ�彦��D��δϴ���ܽ�NaOH���ձ�
E������ʱ���ӿ̶���F������ƿδ���T����������Һ
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶���
���������ж����Ƶ���Һ���ʵ���Ũ�ȴ�С��ɣ���д��ĸ��ƫ�����A��
��5��ijλͬѧ�����Ƶõ�NaOH��Һ����ȡFe��OH��3���壬������ʲô�취Ҳ���ܳɹ���ԭ������Dz���������������Һ�Ʊ� �����������壻��ȷ�ķ���Ӧ���ڷ��ڵ�����ˮ�м��뱥���Ȼ�����Һ������Һ��Ϊ���ɫʱ����ֹͣ���ȣ�
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ�IJ�������ѡ��ʹ�õ�������
��2������ƿ����������һ�����ʵ���Ũ����Һ�Ķ���������ֻ���ڳ�����ʹ�ã���������ʢװ�������ȵ�Һ�壬��������ϡ����Һ����Ϊ��Ӧ������
��3������m=CVM������Ҫ���ʵ�������
��4�������������������ʵ����ʵ�������Һ�������Ӱ�죬����c=$\frac{n}{V}$������������
��5��ʵ�����Ʊ����������������ڷ��ڵ�����ˮ�м��뱥���Ȼ�����Һ��
��� �⣺��1������һ�����ʵ���Ũ�ȵ���Һ�IJ�������Ϊ�����㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ��������У�������ƽ���ձ�������ƿ������Ҫ����������ͷ�ιܣ�
�ʴ�Ϊ������������ͷ�ιܣ�
��2������ƿʱ��������һ�����ʵ���Ũ�ȵıȽϾ��ܵ��������������ȣ�����ƿ���ϱ���ʹ�õ��¶ȣ�������ƿ����ֻ��һ���̶��ߣ�ֻ�����Ƴ����������Ӧ���������Һ������ƿ���ϱ����������ʴ�Ϊ��ace��
��3������0.1mol/LNaOH��Һ450ml��Ӧѡ��500mL����ƿ������500mL��Һ����Ҫ�������Ƶ�����=0.5L��0.1mol/L��40g/mol=2.0g��
�ʴ�Ϊ��2.0��
��4��A������������������룬���³�ȡ�����ʵ�����ƫ�����ʵ����ʵ���ƫ����Һ��Ũ��ƫ��
B����NaOH����ֽ���ϳ����ᳱ�⣬����������NaOH������ƫС�����ʵ����ʵ���ƫС�������Ƴ�����Һ��Ũ��ƫС��
C��������ƿת��ʱ��������Һ�彦�����������ʵ����ʵ���ƫС�������Ƴ�����Һ��Ũ��ƫС��
D��δϴ���ܽ�NaOH���ձ����������ʵ����ʵ���ƫС�������Ƴ�����Һ��Ũ��ƫС��
E������ʱ���ӿ̶��ߣ�������Һ�����ƫ����Һ��Ũ��ƫС��
F������ƿδ���T����������Һ�������ʵ����ʵ�������Һ��������������Ӱ�죬��Һ��Ũ�Ȳ��䣻
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�������Һ�����ƫ����Һ��Ũ��ƫС��
�ʴ�Ϊ��A��
��5��������������Һ���Ȼ�����Ӧ������������������ʵ�����Ʊ����������������ڷ��ڵ�����ˮ�м��뱥���Ȼ�����Һ������Һ��Ϊ���ɫʱ����ֹͣ���ȣ�
�ʴ�Ϊ������������������Һ�Ʊ������������壻�ڷ��ڵ�����ˮ�м��뱥���Ȼ�����Һ������Һ��Ϊ���ɫʱ����ֹͣ���ȣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ������漰����������ѡ���㡢�����������⣬��ȷ����ԭ�������ǽ���ؼ����ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ����Ƭ����Ũ�����У�̽��Al�Ļ����� | |
| B�� | ��NaOH��Һ��������Al2��SO4��3��Һ�У��۲�Al ��OH��3���������ɼ��ܽ� | |
| C�� | ��Al��OH��3��Һֱ�ӵ�����װ����ֽ��©���й��ˣ�ϴ�Ӳ��ռ����� | |
| D�� | ��Al��OH��3����ת���������У�������ϡ���ᣬ�������ɵ���ˮAlCl3���� |
| A�� | H2SO4��BaSO4 | B�� | Cl-��Cl2 | C�� | CuO��Cu | D�� | Fe3+��Fe2+ |
| A�� | MgCl2 | B�� | CH3COONa | C�� | KMnO4 | D�� | FeSO4 |
���������仯ʾ��ͼ�У���ȷ����A������ţ���

��2���ұ��������Ʊ���ϩ��ӦΪ��
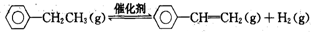
��֪��
| ��ѧ�� | C-H | C-C | C=C | H-H |
| ����/kJ•mol-1 | 412 | 348 | 612 | 436 |
| A�� | ��CuͶ��ϡ������ | |
| B�� | ��Cu�ڳ�ʪ�Ļ��������绯ѧ��ʴ | |
| C�� | ��CuͶ��Ũ�����в����� | |
| D�� | ��Cu���缫��ϡ�������������Һ��� |
| A�� | ��NaHCO3����������Ƶ���ˮ�У�����ɫ���ݣ�H+�� | |
| B�� | ��FeCl2��Һ�еμ�������ˮ���ٵμ�KSCN��Һ�����ֳʺ�ɫ��Cl2�� | |
| C�� | ��FeCl2��Һ�ε���ˮ�����ɵİ�ɫ�����ܿ��ɫ��O2�� | |
| D�� | ��FeCl3��Һ�ε�����-KI��ֽ�ϣ���ֽ������Cl-�� |
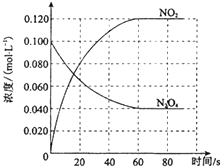 ���ݻ�Ϊ1.00L�������У�ͨ��һ������N2O4��������ӦN2O4��g���T2NO2��g����H�����¶����ߣ�����������ɫ���
���ݻ�Ϊ1.00L�������У�ͨ��һ������N2O4��������ӦN2O4��g���T2NO2��g����H�����¶����ߣ�����������ɫ���