��Ŀ����
19��Ϊ�˱���NO��NO2��N2O4�Դ�������Ⱦ������������������Һ�������մ�������Ӧ����ʽ��2NO2+2NaOH=NaNO2+H2O��NO2+NO+2NaOH=2NaNO2+H2O����������a mol NO��b molNO2��c molN2O4��ɵĻ������ǡ�ñ�VL����������Һ���գ�������ʣ�ࣩ���������������Һ�����ʵ���Ũ��Ϊ��������| A�� | $\frac{a+b+c}{V}$molgL-1 | B�� | $\frac{a+b+2c}{V}$molgL-1 | C�� | $\frac{2a+b+c}{V}$molgL-1 | D�� | $\frac{b+2c}{V}$molgL-1 |
���� �������ǡ�ñ�NaOH��Һ���գ���������ʣ�࣬��������NԪ�ض�ת��Ϊ���Σ���������Na��Nԭ��֮��Ϊ1��1������ԭ���غ����NaOH�����ʵ������ٽ��c=$\frac{n}{V}$����NaOH�����ʵ���Ũ�ȣ�
��� �⣺�������ǡ�ñ�NaOH��Һ���գ���������ʣ�࣬��������NԪ�ض�ת��Ϊ���Σ��ɻ�ѧʽ��֪n��Na��=n��N��������ԭ���غ��֪n��NaOH��=n��NO��+n��NO2��+2n��N2O4��=��a+b+2c��mol��
��c��NaOH��=$\frac{��a+b+2c��mol}{VL}$=$\frac{a+b+2c}{V}$mol/L��
��ѡB��
���� ���⿼������ļ��㣬���ؿ���ѧ��������������������ԭ���غ���м����ܻ���Ϊ����߽���ȷ�ʼ��ٶȣ�ע���᷽ܽ����

��ϰ��ϵ�д�
�����Ŀ
7�� �õ�ⷨ�Ʊ����߷��Ӿۺ���--�ۺ��Ȼ�������ͼ��ʾ����һ�������½��У�����ܷ�
�õ�ⷨ�Ʊ����߷��Ӿۺ���--�ۺ��Ȼ�������ͼ��ʾ����һ�������½��У�����ܷ�
Ӧ�ɱ�ʾ��Al+H2O+AlCl3$\stackrel{���}{��}$[Al2��OH��mClx-m]n+H2����δ��ƽ����������˵������ȷ
���ǣ�������
 �õ�ⷨ�Ʊ����߷��Ӿۺ���--�ۺ��Ȼ�������ͼ��ʾ����һ�������½��У�����ܷ�
�õ�ⷨ�Ʊ����߷��Ӿۺ���--�ۺ��Ȼ�������ͼ��ʾ����һ�������½��У�����ܷ�Ӧ�ɱ�ʾ��Al+H2O+AlCl3$\stackrel{���}{��}$[Al2��OH��mClx-m]n+H2����δ��ƽ����������˵������ȷ
���ǣ�������
| A�� | Cu�缫���Դ�������� | |
| B�� | �ۺ��Ȼ�����ѧʽ��x=4 | |
| C�� | ���ʱ�����ĵ缫��ӦʽΪ��2H++2e?�TH2�� | |
| D�� | ����Դ���ɵ����������缫�������γ�ԭ��� |
14���ߴ��Ⱦ����ǵ��͵����ǽ������ϣ��ֳơ��뵼�塱���ϣ����ķ��ֺ�ʹ��������������һ���������������������з����Ʊ���SiO2 $��_{����}^{��C}$Si���֣�$��_{300��}^{��HCl}$SiHCl3$��_{1000��--1100��}^{�۹���H_{2}}$Si������������˵����ȷ���ǣ�������
| A�� | ����ٵĻ�ѧ����ʽΪ��SiO2+C$\frac{\underline{\;����\;}}{\;}$Si+CO2�� | |
| B�� | ����٢ڢ���ÿ���ɻ�Ӧ1mol Si��ת��4mol���� | |
| C�� | ����������������ᷴӦ�����費��������ᷴӦ | |
| D�� | SiHCl3���е�33.0�棩�к���������SiCl4���е�67.6�棩��ͨ����������ᴿSiHCl3 |
4�������йغ�ˮ�ۺ����õ�˵���У�����ȷ���ǣ�������
| A�� | �������Ӻ�ˮ������Ĺؼ���Ӧ�ǣ�Cl2+2Br-�T2Cl-+Br2 | |
| B�� | ��ˮ�к��м�Ԫ�أ�ֻ�辭�������仯�Ϳ��Եõ��ص��� | |
| C�� | ��ˮ�е�Na+��Cl-�ᾧ��NaCl�Ĺ��̣��γ��˻�ѧ�� | |
| D�� | �Ӻ�ˮ�п��Եõ��Ȼ�þ���پ��������ƽ���þ |
11�������£�NC13��һ����״Һ�壬����ӿռ乹��Ϊ�����Σ����ж�NC13���й�����������ǣ�������
| A�� | NC13��N-C1��������CCl4��C-C1�������� | |
| B�� | NC13�����е�����ԭ�Ӿ��ﵽ8�����ȶ��ṹ | |
| C�� | NCl3�����Ǽ��Է��� | |
| D�� | NBr3�ķе��NCl3�ķе�� |
8����֪M��W��X��Y��Z��Ϊ������Ԫ�أ�MΪ�γɻ�������������Ԫ�أ�W��Zͬ���壬X��Y��Zͬ���ڣ�ZԪ�ص�ԭ������ΪW��2����Z������������ΪY��������������2����Y�ĵ��Ӳ�����������������ȣ�W��X���γ�X2W��X2W2���ֻ��������˵������ȷ���ǣ�������
| A�� | W��X��Y�γɵļ����ӣ���뾶��С��ϵΪW��X��Y | |
| B�� | M��WԪ���γɵļ��⻯����ȶ��ԣ�W��M | |
| C�� | X��ͬ�����н�������ǿ��Ԫ�� | |
| D�� | Z������������Ӧ��ˮ�����Ũ��Һ����Y�ĵ����ڳ����¾��ҷ�Ӧ |
9���л���C5H10Cl2�Ľṹ��ֻ����һ������ͬ���칹���м��֣������������칹����������
| A�� | 4 | B�� | 6 | C�� | 10 | D�� | 18 |
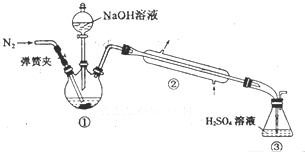 ijС���Է���м��ϡ���ᡢ���ͣ�NH4��2SO4��ҺΪԭ�ϣ�����һϵ�з�Ӧ�Ͳ����ϳ���dz����ɫ����X��Ϊȷ������ɣ���������ʵ�飮
ijС���Է���м��ϡ���ᡢ���ͣ�NH4��2SO4��ҺΪԭ�ϣ�����һϵ�з�Ӧ�Ͳ����ϳ���dz����ɫ����X��Ϊȷ������ɣ���������ʵ�飮