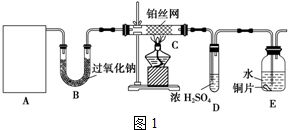
��Ŀ����
20����ӰҺ����Ҫ�ɷ���Na2S2O3�������ܽ⽺Ƥ��Ϊ�й��AgBr���Ӷ�����Ӱ��������1��AgBr��1L1.00mol/LNa2S2O3��Һ���ܽ������Ϊ$\frac{188\sqrt{ab}}{1+2\sqrt{ab}}$g����֪Ksp��AgBr��=a��2Ag++2S2O32-?[Ag��S2O3��2]3-��ƽ�ⳣ��K=b��
��2���ӷ϶�ӰҺ�л������ɲ������·�������һ����������ʹ�϶�ӰҺ�е�������������ʽ�����������ڶ������ٰ���������Ϊ�����ȵ������У�������������ۣ�ʹ�ɵõ�������
��д����һ����Ӧ�����ӷ���ʽ2[Ag��S2O3��2]3-+S2-=Ag2S��+4S2O32-����ڶ���������ж����������壬�ò���Ӧ��ͨ����н���
��3�������õ��ķ����ӷ϶�ӰҺ�л������������µ缫���ϣ�A��ʯī�� B����������ѡ���ǣ��A����B����������A������B���������ĵ缫��ӦʽΪAg++e-=Ag��
���� ��1������AgBr+2S2O32-?[Ag��S2O3��2]3-+Br-�����Ksp��AgBr����-[Ag��S2O3��2]3-��ƽ�ⳣ�����
��2������Ϊǿ�������ȫ���룬������������Ӻ������������Ӧ�������������������ܽ����ȵ������У����Ľ�����ǿ�����������û����������ж����ݴ˷������
��3�����ݵ��ԭ�������жϣ������Ͻ�����������ʧ���ӷ���������Ӧ�������������ӵõ��ӱ�ɵ����������������õ��ķ����ӷ϶�ӰҺ�л�����������Ϊ�����ӵõ����ӣ�
��� �⣺��1����AgBr��1L1.00mol/LNa2S2O3��Һ���ܽ�����ʵ���Ϊx��AgBr+2S2O32-?[Ag��S2O3��2]3-+Br-�����������������ӽ�ϵ�������Ϊx����Һ�е�������Ϊx��Ksp��AgBr��=a��Ksp��AgBr��=c��Ag+��•c��Br-��=a���������������ӽ�ϵ�c��Ag+��=$\sqrt{a}$-x��Ag++2S2O32-?[Ag��S2O3��2]3-��ƽ�ⳣ��K=b��$\frac{C[Ag��{S}_{2}{O}_{3}��_{2}]^{3-}}{C��A{g}^{+}����{C}^{2}��{S}_{2}{{O}_{3}}^{2-}��}$=b��1L1.00mol/LNa2S2O3��Һ��C��S2O32-��=1.00mol/L����$\frac{x}{��\sqrt{a}-x������0.1-2x��^{2}}$=b����ã�x��$\frac{\sqrt{ab}}{1+2\sqrt{ab}}$��AgBrĦ������Ϊ188g/mol������1L1.00mol/LNa2S2O3��Һ���ܽ������Ϊ��$\frac{188\sqrt{ab}}{1+2\sqrt{ab}}$��
�ʴ�Ϊ��$\frac{188\sqrt{ab}}{1+2\sqrt{ab}}$��
��2������Ϊǿ�������ȫ���룬Na2S=2Na++S2-�������Ӻ������������Ӧ��������������2[Ag��S2O3��2]3-+S2-=Ag2S��+4S2O32-���ٰ���������Ϊ�����ȵ������У����Ľ�����ǿ�����������û����������ж����ò�������ͨ����н��У�
�ʴ�Ϊ��2[Ag��S2O3��2]3-+S2-=Ag2S��+4S2O32-��ͨ�����
��3�����أ������������ӵõ��ӱ�ɵ����������������õ��ķ����ӷ϶�ӰҺ�л�����������������������������������������Ag++e-=Ag���ӷ϶�ӰҺ�л�������������������������A��ʯī�����ɣ�
�ʴ�Ϊ��A��B��Ag++e-=Ag��
���� ���⿼���˳����ܽ�ƽ����㡢����ԭ���ķ���Ӧ�ã�Ϊ��Ƶ���㣬������ѧ���ļ��������ͷ��������Ŀ��飬ע������ܶȻ��ļ����Լ�������ķ����жϣ����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

| A�� | �ɱ��������Ȼ��� | B�� | ������Һ�������� | ||
| C�� | ��ˮ�����ռ�Ȼ��� | D�� | ���ᡢ���������������� |
| A�� | ���ʵ�Ħ����������1mol���ʵ����� | |
| B�� | 1molˮ��������ˮ��Ħ��������ˮ����Է�����������ֵ�϶���18 | |
| C�� | �������ʵ�Ħ��������������ͬ | |
| D�� | �����ʵ����������ʵ��������أ�������Է�������һ���������ʵĻ������� |
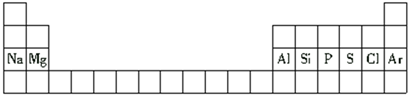
| A�� | ���ǵڶ�����Ԫ�� | |
| B�� | ��ԭ�ӵİ뾶����ԭ�ӵİ뾶С | |
| C�� | �������Ƶļ��Ա�������þ�ļ����� | |
| D�� | ��ԭ�ӵ���������������ԭ�ӵ������������� |
| A�� | һ������ͬ������ | B�� | ������̼ԭ����һ����ͬ | ||
| C�� | �������һ������ͬһͨʽ | D�� | ���ܻ�Ϊͬ���칹�� |
| A�� | c ��Na+����c��HA-����c��H+����c��A2-����c��OH-�� | B�� | c��Na+��+c��H+��=c��HA-��+2c��A2-��+c��OH-�� | ||
| C�� | c ��Na+����c��HA-����c��OH-����c��A2-����c��H+�� | D�� | c��Na+��=c��HA-��+c��HA��+2c��A2-�� |
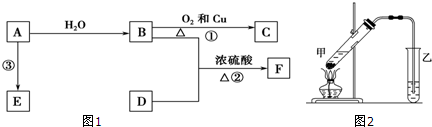
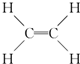 ��B�о������ʵ���Ҫ�����ŵ�����Ϊ�ǻ���
��B�о������ʵ���Ҫ�����ŵ�����Ϊ�ǻ���