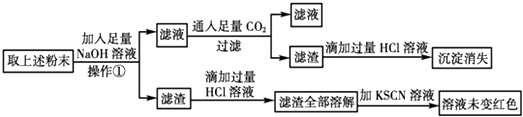
��Ŀ����
����Ŀ������Ԫ�����ڱ���һ���֣�����ÿ��������Ŵ���һ�ֶ�����Ԫ�أ�
�� | ||||||||
�� | �� | |||||||
�� | �� | �� | ||||||
�밴Ҫ��ش��������⣺
��1��Ԫ�آ������ڱ��е�λ��Ϊ
��2���ܡ�������Ԫ����Ƚϣ�������ǿ��������Ԫ�ط��ţ���
��3����ЩԪ�ص�����������Ӧˮ������������ǿ�������ѧʽ����ͬ�����������Ե��� ��
��4��Ԫ�آܺ�Ԫ�آ��γɵĻ�����ĵ���ʽΪ ��
��5��Ԫ�آۺ�Ԫ�آ��⻯�����������ˮ���������������ֱ�պȡ���ǵ�Ũ��Һ����ӽ�ʱ���ɿ��������İ��̣�д������������Ļ�ѧ����ʽ ��
���𰸡�
��1���ڶ����ڵ�IVA��
��2��Na
��3��HClO4��Al��OH��3
��4��![]()
��5��HCl+NH3�TNH4Cl
���������⣺��Ԫ�������ڱ��е�λ��֪���٢ڢۢܢݢֱ���H��C��N��Na��Al��ClԪ�أ���1����Ϊ̼��λ�ڵڶ����ڵ�IVA�壬���Դ��ǣ��ڶ����ڵ�IVA�壻��2��ͬһ����Ԫ���У�Ԫ�صĽ���������ԭ�����������������������Na�Ľ����Դ���Al�����Դ��ǣ�Na����3����ЩԪ�ص�����������Ӧˮ�����У��������������ǿ�����������������ԣ���ѧʽ�ֱ�ΪHClO4��Al��OH��3 �� ���Դ��ǣ�HClO4��Al��OH��3����4��Ԫ�آܺ�Ԫ�آ��γɵĻ�����ΪNaCl��Ϊ���ӻ���������ʽΪ ![]() �����Դ��ǣ�
�����Դ��ǣ� ![]() ����5��Ԫ�آۺ�Ԫ�آ��⻯�����������ˮ���������������ֱ�պȡ���ǵ�Ũ��Һ����ӽ�ʱ���ɿ��������İ��̣�����⻯����NH3 �� ���⻯����HCl�����߷�Ӧ�����Ȼ�泥���Ӧ����ʽΪHCl+NH3�TNH4Cl�����Դ��ǣ�HCl+NH3�TNH4Cl��
����5��Ԫ�آۺ�Ԫ�آ��⻯�����������ˮ���������������ֱ�պȡ���ǵ�Ũ��Һ����ӽ�ʱ���ɿ��������İ��̣�����⻯����NH3 �� ���⻯����HCl�����߷�Ӧ�����Ȼ�泥���Ӧ����ʽΪHCl+NH3�TNH4Cl�����Դ��ǣ�HCl+NH3�TNH4Cl��

 ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д� ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�����Ŀ����ʯī�缫������й��ʵ�顣
ʵ��һ | ʵ��� | |
װ�� |
|
|
a��d����ֽ������b����죬�ֲ���ɫ��c�������Ա仯 | ����ʯī�缫���������ݲ�����n�������ݲ��������� |
���ж�ʵ������Ľ��ͻ��Ʋⲻ�������ǣ� ��
A. a��d����2H2O+2e-=H2��+2OH- B. b��ʧ���ӣ�����������Ӧ
C. c�������˷�Ӧ��Fe-3e-=Fe3+ D. ����ʵ��һ��ԭ����ʵ�����m��������ͭ