��Ŀ����
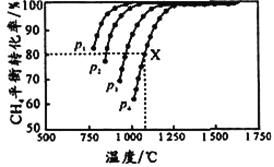
����Ŀ��һ���¶��£���1L�����ܱ������м���lmol��N2(g)��3molH2(g)������Ӧ��N2(g)+3H2(g) ![]() 2NH3(g) ��H<0�� NH3�����ʵ�����ʱ��Ĺ�ϵ���±���ʾ������˵������ȷ����( )
2NH3(g) ��H<0�� NH3�����ʵ�����ʱ��Ĺ�ϵ���±���ʾ������˵������ȷ����( )
ʱ��(min) | 0 | t1 | t2 | t3 |
NH3���ʵ���(mol) | 0 | 0.2 | 0.3 | 0.3 |
A. 0t1min��v(NH3)=0.2/t1mol��L-1��min-1
B. t3ʱ�ټ���1mol��N2(g)��3molH2(g)����Ӧ����ƽ��ʱ��c(N2)>0.85mol��L-1
C. N2(g)+3H2(g) ![]() 2NH3(g)�Ļ��С��2NH3(g)
2NH3(g)�Ļ��С��2NH3(g) ![]() N2(g)+3H2(g)�Ļ��
N2(g)+3H2(g)�Ļ��
D. �����¶ȣ���ʹ����Ӧ���ʼ�С���淴Ӧ��������ƽ������
���𰸡�D
��������
A. 0t1minʱ����NH3��Ũ��=![]() =0.2mol/L������NH3��ƽ����Ӧ����=
=0.2mol/L������NH3��ƽ����Ӧ����=![]() =
=![]() mol��L-1��min-1��A����ȷ��
mol��L-1��min-1��A����ȷ��
B.�ɱ������ݿ�֪����t3ʱ��Ӧ�Ѵ���ƽ��״̬�����ݷ�Ӧ����ʽN2(g)+3H2(g) ![]() 2NH3(g)��֪��ʱ����N2�����ʵ���=
2NH3(g)��֪��ʱ����N2�����ʵ���=![]()
![]() =
=![]() ��0.3mol=0.15mol������ƽ��ʱN2��Ũ��c(N2)=
��0.3mol=0.15mol������ƽ��ʱN2��Ũ��c(N2)=![]() =0.85mol/L����t3ʱ�ټ���1mol��N2(g)��3molH2(g)ʱ������ƽ��������Ӧ�����ƶ����������1molN2��������ȫת���������ٴ�ƽ��ʱc(N2)>0.85mol/L��B����ȷ��
=0.85mol/L����t3ʱ�ټ���1mol��N2(g)��3molH2(g)ʱ������ƽ��������Ӧ�����ƶ����������1molN2��������ȫת���������ٴ�ƽ��ʱc(N2)>0.85mol/L��B����ȷ��
C. ��Ϊ�ϳɰ���ӦN2(g)+3H2(g)![]() 2NH3(g)�Ƿ��ȷ�Ӧ����������ϵͼΪ��
2NH3(g)�Ƿ��ȷ�Ӧ����������ϵͼΪ��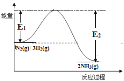 ������E1Ϊ����Ӧ�Ļ�ܣ�E2Ϊ�淴Ӧ�Ļ��������Ȼ�ϳɰ��Ļ�ܣ�E1��С�����淴Ӧ2NH3(g)
������E1Ϊ����Ӧ�Ļ�ܣ�E2Ϊ�淴Ӧ�Ļ��������Ȼ�ϳɰ��Ļ�ܣ�E1��С�����淴Ӧ2NH3(g) ![]() N2(g)+3H2(g)�����E2����C����ȷ��
N2(g)+3H2(g)�����E2����C����ȷ��
D.�����¶Ȳ����Ƿ�Ӧ����ӻ�����������Ӷ����������������Ӧ���淴Ӧ�Ļ���Ӱٷ�������������������Ӧ���ʺ��淴Ӧ���ʶ������������ȷ�Ӧ��������ij̶ȴ��ڷ��ȷ�Ӧ��������ij̶ȣ�����ƽ�������ȷ�Ӧ�����ƶ������÷�Ӧ���淴Ӧ�����ƶ���D�����ѡD��