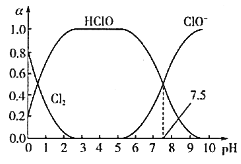
��Ŀ����
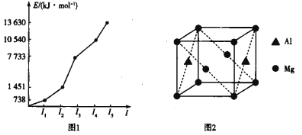
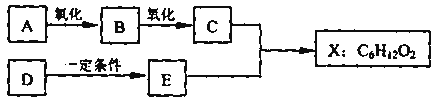
����Ŀ������ͼ��X����֧���ġ����й���ζ�ĺϳ����ϣ������ڵ�����ֹ������㾫����֪
D�ڱ�״���µ��ܶ�Ϊ1.25 g/L�������������������һ������ʯ�ͻ�����չˮƽ��E������
�г�����һ���л�������ʼ�ת����ϵ���£�
��ش��������⡣
(1)A��������________________________��
(2)B��������������________________��
(3)C+E![]() X�Ļ�ѧ��Ӧ������___________________��Ӧ��
X�Ļ�ѧ��Ӧ������___________________��Ӧ��
(4)д������������A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ (����A)��_________��
(5)X������������Һ��Ӧ�Ļ�ѧ����ʽ��______________________________��
(6)��DΪԭ������һ�ֳ������ϵĻ�ѧ����ʽ��___________________________________��
���𰸡�1������ ȩ�� ������Ӧ����ȡ����Ӧ�� (CH3)3COH��CH3CH2CH(OH)CH3��(CH3)2CHCH2OH CH3CH2CH2COOCH2CH3��NaOH![]() CH3CH2CH2COONa��CH3CH2OH
CH3CH2CH2COONa��CH3CH2OH ![]()
��������
D�ڱ�״���µ��ܶ�Ϊ1.25g/L������Է�������Ϊ1.25��22.4=28�������������������һ������ʯ�ͻ�����չˮƽ��ӦΪCH2=CH2��A�������������ֱ�����B��C����AӦΪ����BΪȩ��CΪ�ᣬ��X����֧���ġ����й���ζ�ĺϳ����ϣ�ӦΪ��������EΪ����ӦΪCH3CH2OH����XΪCH3CH2CH2COOCH2CH3��CΪCH3CH2CH2COOH��BΪCH3CH2CH2CHO��AΪCH3CH2CH2CH2OH����϶�Ӧ�л���Ľṹ�������Լ���ĿҪ��ɽ����⡣
D�ڱ�״���µ��ܶ�Ϊ1.25g/L������Է�������Ϊ1.25��22.4=28�������������������һ������ʯ�ͻ�����չˮƽ��ӦΪCH2=CH2��A�������������ֱ�����B��C����AӦΪ����BΪȩ��CΪ�ᣬ��X����֧���ġ����й���ζ�ĺϳ����ϣ�ӦΪ��������EΪ����ӦΪCH3CH2OH����XΪCH3CH2CH2COOCH2CH3��CΪCH3CH2CH2COOH��BΪCH3CH2CH2CHO��AΪCH3CH2CH2CH2OH��
��1��AΪCH3CH2CH2CH2OH��Ϊ1-�������ʴ�Ϊ��1-������
��2��BΪCH3CH2CH2CHO�����еĹ�����Ϊȩ�����ʴ�Ϊ��ȩ����
��3�������Ϸ�����֪C+E��X�Ļ�ѧ��Ӧ������������ȡ������Ӧ���ʴ�Ϊ��������ȡ������
��4��AΪCH3CH2CH2CH2OH����Ӧ��ͬ���칹��(CH3)3COH��CH3CH2CH(OH)CH3��(CH3)2CHCH2OH�ȣ�
�ʴ�Ϊ��(CH3)3COH��CH3CH2CH(OH)CH3��(CH3)2CHCH2OH��������д��������
��5��XΪCH3CH2CH2COOCH2CH3����NaOH��Һ�ɷ���ˮ�⣬��Ӧ�ķ���ʽΪCH3CH2CH2COOCH2CH3��NaOH![]() CH3CH2CH2COONa��CH3CH2OH��
CH3CH2CH2COONa��CH3CH2OH��
�ʴ�Ϊ��CH3CH2CH2COOCH2CH3��NaOH![]() CH3CH2CH2COONa��CH3CH2OH��
CH3CH2CH2COONa��CH3CH2OH��
��6��DΪCH2=CH2���ɷ����Ӿ۷�Ӧ����Ӧ�ķ���ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����ǰ����ڷ��ǻ��д��ڼ�������¿��Է���ż����Ӧ�����Ѽ�����Ӧ�������ºͣ�������Ŀ��������ʸߡ����������ø÷����ϳɻ�����H��һ�ַ�����
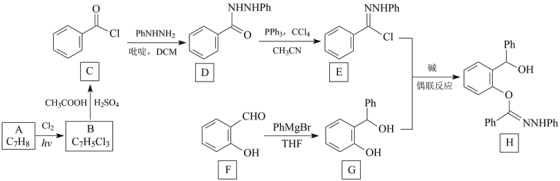
ע�����Ϻϳ�·���У�Ph������������PhNHNH2��ʾ![]() ��
��
ʵ�� | �� | �¶�/�� | �ܼ� | ����% |
1 | Et3N | 25 | DCM | <10 |
2 | ��� | 25 | DCM | <5 |
3 | Cs2CO3 | 25 | DCM | 70 |
4 | LiOtBu | 25 | DCM | 43 |
5 | Cs2CO3 | 25 | DMF | 79 |
6 | Cs2CO3 | 25 | CH3CN | 83 |
�ش��������⣺
��1��A�Ļ�ѧ����Ϊ_________��B�Ľṹ��ʽΪ___________��
��2����C����D�ķ�Ӧ������________��F�еĹ�����������___________��
��3��H�ķ���ʽΪ_______________��
��4����ͬ������ż����Ӧ����H���ʵ�Ӱ�����ϱ���
�۲��ϱ���֪����ѡ��DCM���ܼ�ʱ�������˵ļ���____________����ʵ��3��ʵ��4��ʵ��5�ɵó��Ľ����ǣ�25��ʱ��____________________��H������ߡ�
��5��XΪG��ͬ���칹�壬д����������������X�Ľṹ��ʽ��____________��
����������������G��ͬ���������ֲ�ͬ��ѧ�������⣬�������Ϊ1:1:2:2
��6������ż����Ӧ����![]() �ͻ�����DΪԭ���Ʊ�
�ͻ�����DΪԭ���Ʊ� ��
��
д���ϳ�·�ߣ�____________________________���������Լ���ѡ��