��Ŀ����
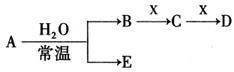
A��J���ճ������г��������ֽ����������ֽ�����NaOH���ԭ��أ�A��������F�����������嵥�ʣ������������µ�ת����ϵ�����ֲ��P������ȥ����Ks5u
��ش��������⣺
��1��д����ԭ��ص��ܷ�Ӧ����ʽ_____________________��
��2��д���ڵĻ�ѧ����_________________��
��3������ʱpH=12��C��Һ�У����ʵ������������ʵ�������Ũ��֮��Ϊ ����д������ʽ��
��4��������J��������Ϊ�����������ÿ����1mol J�ų�Q kJ����������д��A��J��Ӧ���Ȼ�ѧ����ʽ ��
��1��2Al �� 2NaOH +6H2O="2Na" [Al(OH)4]+ 3 H2��
��2��4NH3+5O2  4NO+6H2O
4NO+6H2O
��3����10-2�C10-12��mol·L��1����c(OH��)�Cc(H��)
��4��3Fe3O4(S) + 8Al(S) == 9Fe (S)+4 Al2O3(S) ��H=��9Q kJ·mol��1
���������������1�����������֪��A��Al;J��Fe.�������ֽ�����NaOH���ԭ��أ�Al������,Fe����������ԭ��ص��ܷ�Ӧ����ʽ2Al �� 2NaOH +6H2O="2Na" [Al(OH)4]+ 3 H2��C��NaAlO2,D��H2��E��Al(OH)3,H��Al2O3,M��FeS��F��N2��G��NH3��I��NO��K��NO2��L��HNO3����2���ڵĻ�ѧ����ʽΪ��4NH3+5O2  4NO+6H2O����3������ʱpH=12��C��Һ�У�C(H+)=1��10-12mol/L.C(OH-)=Kw��C(H+)=(1��10-14)��(1��10-12)=1��10-2mol/L������Һ�ʵ����ԡ���C(Na+)+C(H+)=C(AlO2-)+C(OH-),C(Na+)-C(AlO2-)=C(OH-)-C(H+)=(10-210-12)mol/L.��4����������Fe3O4��Ӧ���Ȼ�ѧ����ʽΪ��3Fe3O4(S) + 8Al(S) == 9Fe (S)+4 Al2O3(S) ��H=��9Q kJ·mol��1
4NO+6H2O����3������ʱpH=12��C��Һ�У�C(H+)=1��10-12mol/L.C(OH-)=Kw��C(H+)=(1��10-14)��(1��10-12)=1��10-2mol/L������Һ�ʵ����ԡ���C(Na+)+C(H+)=C(AlO2-)+C(OH-),C(Na+)-C(AlO2-)=C(OH-)-C(H+)=(10-210-12)mol/L.��4����������Fe3O4��Ӧ���Ȼ�ѧ����ʽΪ��3Fe3O4(S) + 8Al(S) == 9Fe (S)+4 Al2O3(S) ��H=��9Q kJ·mol��1
���㣺�����ε�ˮ�⡢���ȷ�Ӧ��ԭ��ط�Ӧ��֪ʶ��

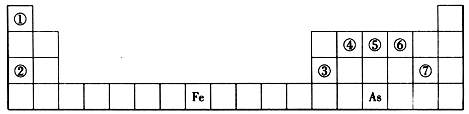
�Т١��ڡ��ۡ��ܡ��ݡ��ޡ��ߡ��ࡢ�ᡢ��ʮ��Ԫ�أ�ԭ�������������ᡢ��ڵ������ڣ������Ϊ������Ԫ�ء�
��1�����ڡ��ߡ�������Ԫ�������ڱ������λ������
| �� | | | |
| | | �� | �� |
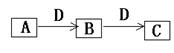
������γɵ�Һ�廯�����dz������ܼ������Ԫ�������ڱ��е�λ���� ����Ԫ�ص�������ĵ���ʽ�� ��������γɵĻ������д��ڵĻ�ѧ���� ����ڡ��ߡ�������Ԫ������������Ӧ��ˮ��������������ǿ��˳���� (�û�ѧʽ��ʾ)���ߡ�������Ԫ���γɵ������Ӱ뾶��С˳���� (�����ӷ�����)��
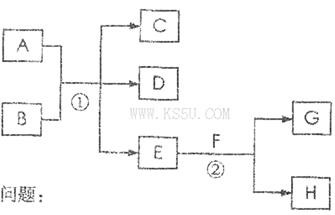
��2�����ס����������������Ϊ����������Ԫ������ɵĵ��ʻ���Ҽס��ҡ���Ϊ��ɫ���壬��Ϊ����ɫ���塣������ͼ��ʾת����ϵ�Ʋ⣺
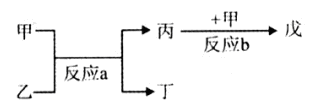
��д����Ӧa�Ļ�ѧ����ʽ�� ��
��д�����붡��Ӧ�Ļ�ѧ����ʽ�� ��
��3������ͼ��A��B��C��D��E�ֱ�������10��Ԫ����ɵĵ��ʻ��
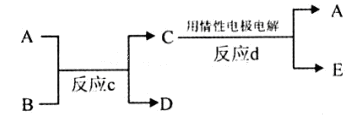
������Ӧc����A�����ص����������B��ˮ��Һ������A�Ǻ�ɫ�������ʣ�E�Ǿ��л���ɫ�����塣��Ӧd�������ĵ缫��ӦʽΪ�� ��
������Ӧc���ڸ����½��еġ�����B�ǰ���ɫ���壬������C��һ�����Ի������Ӧc�Ļ�ѧ����ʽΪ ��
����ʵ���У����ӹ��������ȫ�ܽ����
| A����H2O2��Һ�м�������MnO2��ĩ |
| B����һС����ƬͶ������NaOH��Һ�� |
| C��������������������������Ũ��ˮ�� |
| D�������½�һС����ƬͶ��������Ũ������ |