��Ŀ����
����Ŀ��������ЧӦ����ȫ���ע�Ļ�������֮һ��C02��Ŀǰ�����к�����ߵ�һ���������壬C02���ۺ������ǽ�����Ҽ���Դ�������Ч;����
(1)�о�����C02��H2�ڴ��������¿ɷ�����Ӧ����CH3OH����֪���ַ�Ӧ���Ȼ�ѧ����ʽ���£�CH3OH��g��+![]() O2��g��= CO2��g��+2H2O��g����H1=akJmol-��H2��g��+
O2��g��= CO2��g��+2H2O��g����H1=akJmol-��H2��g��+![]() O2��g��=H2O��l����H2����CO2��g��+3H2��g��= CH3OH��g��+2H2O��l����H=___kJmol-��
O2��g��=H2O��l����H2����CO2��g��+3H2��g��= CH3OH��g��+2H2O��l����H=___kJmol-��
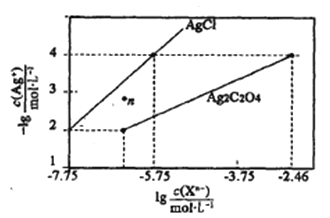
(2)C02������Ҳ�ܺϳɵ�̼ϩ����2C02(g)+6H2(g) =C2H4(g)+4H20(g)����ͬ�¶��� ƽ��ʱ��������̬���ʵ����ʵ�����ͼ��ʾ������c��ʾ������Ϊ____(�ѧʽ)��

(3)C02��H2�ڴ���Cu/ZnO�����¿ɷ�������ƽ�з�Ӧ���ֱ�����CH3OH��CO��
��ӦA: C02(g)+3H2(g) = CH30H(g)+H20(g)
��ӦB: C02(g)+H2(g)=C0(g)+H20(g)
����C02��H2��ʼͶ�ϱ�Ϊ1 : 3ʱ���¶ȶ�C02ƽ��ת���ʼ��״���CO���ʵ�Ӱ����ͼ��ʾ��
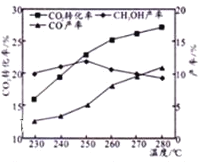
����ͼ��֪�¶�����CO�IJ�������������Ҫԭ�������________��
����ͼ��֪��ȡCH3OH�����˵��¶���____________�����д�ʩ�������C02ת��ΪCH3OH��ƽ��ת���ʵ���______(����ĸ)��
A.ʹ�ô��� B.������ϵѹǿ C.����C02�ͳ��ij�ʼͶ�ϱ�
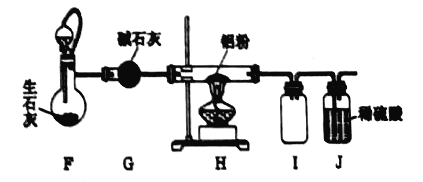
(4)�ڴ�������ͨ��ʩ�ӵ�ѹ�ɽ��ܽ���������Һ�еĶ�����ֱ̼��ת��Ϊ�Ҵ��������� �Ҵ��ĵ缫��ӦʽΪ_________��
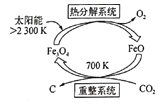
(5)��C02��ȡC��̫���ܹ�����ͼ��ʾ�����ȷֽ�ϵͳ�������ķ�ӦΪ 2Fe304 ![]() 6Fe0+02�������ֽ�1mo;Fe304ת�Ƶ��ӵ����ʵ���Ϊ______��������ϵͳ��������Ӧ�Ļ�ѧ����ʽΪ_______��
6Fe0+02�������ֽ�1mo;Fe304ת�Ƶ��ӵ����ʵ���Ϊ______��������ϵͳ��������Ӧ�Ļ�ѧ����ʽΪ_______��
���𰸡�3b��a C2H4 ��ӦB����Ӧ�����ȷ�Ӧ���¶�����ƽ�������ƶ���CO�������� 250�� AC 2CO2��12H����12e��=C2H5OH��3H2O 2 mol 6FeO+CO2![]() 2Fe3O4+C
2Fe3O4+C
��������
��֪��CH3OH(g)+![]() O2(g)�TCO2(g)+2H2O(1)��H1=akJmol-1����H2(g)+
O2(g)�TCO2(g)+2H2O(1)��H1=akJmol-1����H2(g)+![]() O2(g)=H2O(1)��H2=bkJmol-1�����ݸ�˹����֪������3-�ٵã�CO2(g)+3H2(g)CH3OH(g)+H2O(g)��
O2(g)=H2O(1)��H2=bkJmol-1�����ݸ�˹����֪������3-�ٵã�CO2(g)+3H2(g)CH3OH(g)+H2O(g)��
(2)����ͼ֪�������¶ȣ��������ʵ�������˵��ƽ�������ƶ���������Ӧ�Ƿ��ȷ�Ӧ��a���������¶����ߣ����ʵ�������Ϊ������̼��b��c�����¶����������ʵ������ͣ�Ϊ������ˮ����ϩ����ˮ�ı仯��������ϩ���ݴ��ж�c���ߴ������ʣ�
(3)�ٷ�ӦB����Ӧ�����ȷ�Ӧ���¶�����ƽ�������ƶ���CO�������ߣ�
�ھ�ͼʾ���з���250��״�ת������ߣ���CO2(g)+3H2(g)CH3OH(g)+H2O(g)��H1=-53.7kJmol-8 ��֪���CO2ת��ΪCH3OHƽ��ת���ʣ�Ӧʹƽ���������ƶ����ɽ����¶ȣ�����Ũ�ȣ�
(4)���ʱ��������̼��b���ϵõ��ӷ�����ԭ��Ӧ�����Ҵ���
(5)�÷�Ӧ��FeԪ�ػ��ϼ���+3�۱�Ϊ+2�ۣ�OԪ�ػ��ϼ���-2�۱�Ϊ0�ۣ�����ת�Ƶ��Ӻ�Fe3O4֮��Ĺ�ϵʽ���㣻����ͼ֪����Ӧ���Ƕ�����̼��FeO���������������������C����Ӧ������700K��
(1)��֪��CH3OH(g)+![]() O2(g)�TCO2(g)+2H2O(1)��H1=akJmol-1����H2(g)+
O2(g)�TCO2(g)+2H2O(1)��H1=akJmol-1����H2(g)+![]() O2(g)=H2O(1)��H2=bkJmol-1�����ݸ�˹����֪������3-�ٵã�CO2(g)+3H2(g)CH3OH(g)+H2O(g))������H=(3b-a)kJmol-1��
O2(g)=H2O(1)��H2=bkJmol-1�����ݸ�˹����֪������3-�ٵã�CO2(g)+3H2(g)CH3OH(g)+H2O(g))������H=(3b-a)kJmol-1��
(2)����ͼ֪�������¶ȣ��������ʵ�������˵��ƽ�������ƶ���������Ӧ�Ƿ��ȷ�Ӧ��a���������¶����ߣ����ʵ�������Ϊ������̼��b��c�����¶����������ʵ������ͣ�Ϊ������ˮ����ϩ����ˮ�ı仯��������ϩ������b���ߴ���H2O��c���ߴ�����ϩ��
(3)����ͼ2��֪�¶�����CO�IJ�������������Ҫԭ������Ƿ�ӦB����Ӧ�����ȷ�Ӧ���¶�����ƽ�������ƶ���CO�������ߣ�
(3)�ھ�ͼʾ���з���250��״�ת������ߣ��ʻ�ȡCH3OH�����˵��¶���250�棻
A��ʹ�ô�����ƽ�ⲻ�ƶ����������ת���ʣ���A����
B��������ϵѹǿ��ƽ�����������ƶ�����״�ת���ʣ���B��ȷ��
C������CO2��H2�ij�ʼͶ�ϱȣ�������������ת���ʣ�������̼��ת���ʼ�С����C����
�ʴ�ΪAC��
(4)���ʱ��������̼��b���ϵõ��ӷ�����ԭ��Ӧ����C2H5OH���缫��ӦʽΪ2CO2+12H++12e--=C2H5OH+3H2O��
(5)�÷�Ӧ��FeԪ�ػ��ϼ���+3�۱�Ϊ+2�ۣ�OԪ�ػ��ϼ���-2�۱�Ϊ0�ۣ��ֽ�2mol����������ת��4mol���ӣ���ֽ�1mol����������ת��2mol���ӣ�����ͼ֪����Ӧ���Ƕ�����̼��FeO���������������������C����Ӧ������700K����Ӧ����ʽΪ6FeO(S)+CO2![]() 2Fe3O4(S)+C��
2Fe3O4(S)+C��

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�����Ŀ����0.1320mol/L��HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ��ʵ���������±���ʾ��
ʵ���� | ����NaOH��Һ�����/mL | HCl��Һ�����/mL |
1 | 25.00 | 24.41 |
2 | 25.00 | 24.39 |
3 | 25.00 | 24.60 |
�ش��������⣺
��1����ͼ�м�Ϊ___________�ζ��ܣ���Ϊ_________ �ζ��ܣ����ʽ����ʽ ����
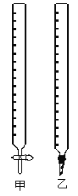
��2��ʵ���У���Ҫ��ϴ�������ǣ�________________________
��3��ȡ����ҺNaOH��Һ25.00ml ����ƿ�У�ʹ�÷�̪��ָʾ�����ζ��յ���ж�������________________________________________
��4�����ζ�ǰ���ζ��ܼ�������ݣ��ζ���������ʧ����ʹ������____________���ƫ�ߡ���ƫ�͡������䡱����ͬ����������ʽ�ζ��ܶ���ʱ���ζ�ǰ���Ӷ������ζ�����ȷ��������������___________��
��5�� δ֪Ũ�ȵ�NaOH��Һ�����ʵ���Ũ��Ϊ_____________mol/l��