��Ŀ����
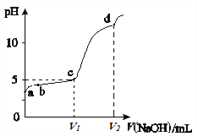
����Ŀ��������ʵ���Ũ�ȵ�K2S��KOH�����Һ�еμ�ϡ������������������Ҫ��������֣�H2S��HS��S2���ķֲ�������ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ�������μ���������Ĺ�ϵ��ͼ��ʾ�����Եμӹ���H2S������ݳ���������˵������ȷ����
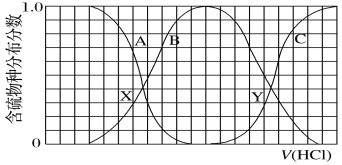
A.A���߱�ʾS2-���������������ʱ�ķֲ������ı����
B.X��YΪ����������㡣����֪Y�㴦��pH����ɼ���Ka1��H2S��
C.X��Y���Ӧ��Һ��ˮ�ĵ���̶ȴ�С��ϵΪ��X<Y
D.Y���Ӧ��Һ��c��K�����뺬�����Ũ�ȵĴ�С��ϵΪ��c��K������3[c��H2S����c��HS����c��S2��]
���𰸡�C
��������
�μ�����ʱ�������Ⱥ�KOH��Ӧ��Ȼ���ٺ�K2S��Ӧ�����ȷ���S2-+H+=HS-���ù�����S2-�������٣�HS-����������Ȼ����HS-+H+=H2S����ʱHS-�����½���H2S����������A����S2-��B����HS-�� C����H2S��
A�����ݷ�����֪A���߱�ʾS2-���������������ʱ�ķֲ������ı��������A��ȷ��
B��Y���ʾc(H2S)= c(HS)��Ka1(H2S)=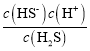 ����c(H2S)= c(HS)ʱ��Ka1(H2S)=c(H+)����������֪Y�㴦��pH����ɼ���Ka1(H2S)����B��ȷ��
����c(H2S)= c(HS)ʱ��Ka1(H2S)=c(H+)����������֪Y�㴦��pH����ɼ���Ka1(H2S)����B��ȷ��
C��X��c(HS-)=c(S2-)��Y��c(H2S)=c(HS-)��S2-��HS-��ˮ��ٽ�ˮ�ĵ��룬H2SΪ������ˮ���룬��X��ˮ�ĵ���̶Ƚϴ�C����
D��ԭ��ҺΪ�����ʵ���Ũ�ȵ�K2S��KOH�����Һ�����������غ��֪c(K��)��3[c(H2S)��c(HS)��c(S2)]����D��ȷ��
�ʴ�ΪC��

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�����Ŀ����������(ClNO)���л��ϳ��е���Ҫ�Լ�������NO��Cl2��ͨ�������·�Ӧ�õ�����ѧ����ʽΪ2NO(g)��Cl2(g)![]() 2ClNO(g)��
2ClNO(g)��
(1)���������������ڴ����еĺ������������ʱ�������������ȣ��漰���·�Ӧ���� 2NO2(g)��NaCl(s)![]() NaNO3(s)��ClNO(g) K1����4NO2(g)��2NaCl(s)
NaNO3(s)��ClNO(g) K1����4NO2(g)��2NaCl(s)![]() 2NaNO3(s)��2NO(g)��Cl2(g) K2����2NO(g)��Cl2(g)
2NaNO3(s)��2NO(g)��Cl2(g) K2����2NO(g)��Cl2(g)![]() 2ClNO(g) K3,��K1��K2��K3֮��Ĺ�ϵΪK3=_______________����K1��K2��ʾ����
2ClNO(g) K3,��K1��K2��K3֮��Ĺ�ϵΪK3=_______________����K1��K2��ʾ����
(2)��֪���ֻ�ѧ���ļ����������±������������ȵĽṹΪCl��N==O��
��ѧ�� | N��O | Cl��Cl | Cl��N | N==O |
����/(kJ��mol��1) | 630 | 243 | a | 607 |
��2NO(g)��Cl2(g)![]() 2ClNO(g)�ķ�Ӧ�� ��H��a�Ĺ�ϵΪ ��H = ___kJ��mol��1��
2ClNO(g)�ķ�Ӧ�� ��H��a�Ĺ�ϵΪ ��H = ___kJ��mol��1��
(3)300��ʱ��2NO(g)��Cl2 (g)![]() 2ClNO(g)������Ӧ���ʱ���ʽΪv�� = k��cn (ClNO)��������ʺ�Ũ�ȵĹ�ϵ���±���
2ClNO(g)������Ӧ���ʱ���ʽΪv�� = k��cn (ClNO)��������ʺ�Ũ�ȵĹ�ϵ���±���
��� | c(ClNO)/(mol��L��1) | v/(mol��L��1��s��l) |
�� | 0. 30 | 3. 60��10��9 |
�� | 0. 60 | 1. 44��10��8 |
�� | 0. 90 | 3. 24��10��8 |
n = ___��k = ____��
(4)������Ⱥ����ܱ������г������ʵ���֮��Ϊ2 : 1��NO��Cl2���з�Ӧ2NO(g)��Cl2(g)![]() 2ClNO(g)�����жϷ�Ӧ�Ѵﵽ��ѧƽ��״̬����__�����ţ���
2ClNO(g)�����жϷ�Ӧ�Ѵﵽ��ѧƽ��״̬����__�����ţ���
a�������е�ѹǿ���� b��2v��(NO) = v��(Cl2)c�������ƽ����Է����������ֲ��� d���÷�Ӧƽ�ⳣ�����ֲ���e��NO��Cl2������ȱ��ֲ���
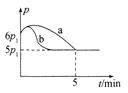
(5)25��ʱ�������Ϊ2 L�Ҵ���ѹ�Ƶĺ����ܱ�������ͨ��0.08 mol NO��0.04 mol Cl2������Ӧ��2NO(g)��Cl2 (g)![]() 2ClNO(g)��H��
2ClNO(g)��H��
������Ӧ��ʼ��ƽ��ʱ�¶���ͬ����÷�Ӧ������ѹǿ(p)��ʱ��(t)�ı仯��ͼ������a��ʾ���� ��H __���>����<����ȷ������0��������������ͬ�����ı�ijһ����ʱ�������ѹǿ(p)��ʱ��(t)�ı仯��ͼ������b��ʾ����ı��������______��
��ͼ�Ǽס���ͬѧ���������Ӧƽ�ⳣ���Ķ���ֵ(lg K)���¶ȵı仯��ϵ��������ȷ��������____����ס����ҡ�����mֵΪ_____��