��Ŀ����
20��д�����л�ѧ��Ӧ�����ӷ���ʽ����1����H2SO4��Һ������Ba��OH��2��Һ�����ԣ�д����Ӧ�����ӷ���ʽ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O��
��2��̼�����ϡ���ᷴӦ�����ӷ���ʽCaCO3+2H+=CO2��+H2O+Ca2+
��3����д��Na2SO4�ĵ��뷽��ʽNa2SO4�T2Na++SO42-��
��������������������Һ��Ӧ�����ӷ�Ӧ����ʽAl2O3+2OH-�T2AlO2-+H2O
��OH-+H+�TH2O��дΪ��ѧ����ʽH2SO4+2NaOH�T2H2O+Na2SO4
��4������500mL 0.5mol/L��ϡ���ᣬ��Ҫ98%���ܶ�Ϊ1.84g/cm3����Ũ����13.6 mL����������ϡ������Һ������������Ͳ���ձ������������������������Ҫ500mL����ƿ����ͷ�ιܣ�
��5��ʵ������ʢ���ռ���Һ���Լ�ƿ�����ò�����������Ϊ���û�ѧ����ʽ��ʾ��2NaOH+SiO2�TNa2SiO3+H2O��
���� ��1�������ԣ���Ӧ�������ᱵ��ˮ��
��2����Ӧ�����Ȼ��ơ�ˮ��������̼��
��3����Ϊǿ����ʣ���ȫ����������Ӻ���������ӣ�
�ڷ�Ӧ����ƫ�����ƺ�ˮ��
��OH-+H+�TH2O��ʾǿ����ǿ�Ӧ���ɿ������κ�ˮ�����ӷ�Ӧ��
��4�����c=$\frac{1000��w}{M}$������ǰ����Һ�����ʵ����ʵ���������㣻������Һһ����Ҫ����ƿ��
��5���ռ��벣���еĶ������跴Ӧ���ɹ����ƺ�ˮ�������Ƶ�ˮ��Һ����ճ���ԣ�
��� �⣺��1�������ԣ���Ӧ�������ᱵ��ˮ�����ӷ�ӦΪBa2++2OH-+2H++SO42-=BaSO4��+2H2O���ʴ�Ϊ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O��
��2����Ӧ�����Ȼ��ơ�ˮ��������̼�����ӷ�ӦΪCaCO3+2H+=CO2��+H2O+Ca2+���ʴ�Ϊ��CaCO3+2H+=CO2��+H2O+Ca2+��
��3����Ϊǿ����ʣ���ȫ����������Ӻ���������ӣ����뷽��ʽΪNa2SO4�T2Na++SO42-���ʴ�Ϊ��Na2SO4�T2Na++SO42-��
�ڷ�Ӧ����ƫ�����ƺ�ˮ�����ӷ�ӦΪAl2O3+2OH-�T2AlO2-+H2O���ʴ�Ϊ��Al2O3+2OH-�T2AlO2-+H2O��
��OH-+H+�TH2O��ʾǿ����ǿ�Ӧ���ɿ������κ�ˮ�����ӷ�Ӧ����H2SO4+2NaOH�T2H2O+Na2SO4���ʴ�Ϊ��H2SO4+2NaOH�T2H2O+Na2SO4��
��4������ǰ����Һ�����ʵ����ʵ������䣬��0.5L��0.5mol/L=$\frac{1000��1.84��98%}{98}$��V��֪��V=13.6mL��������Һһ����Ҫ500mL����ƿ�����ݻ���Ҫ��ͷ�ιܣ�
�ʴ�Ϊ��13.6��500mL����ƿ����ͷ�ιܣ�
��5���ռ��벣���еĶ������跴Ӧ���ɹ����ƺ�ˮ�������Ƶ�ˮ��Һ����ճ���ԣ���ӦΪ2NaOH+SiO2�TNa2SiO3+H2O���ʴ�Ϊ��2NaOH+SiO2�TNa2SiO3+H2O��
���� ���⿼�����ӷ�Ӧ����ʽ��д�������жϣ�Ϊ��Ƶ���㣬���շ����ķ�Ӧ�����ӷ�Ӧ����д����Ϊ���Ĺؼ������ظ��ֽⷴӦ�������йص����ӷ�Ӧ��������Ӧ�������Ŀ��飬ע�����ӷ�Ӧ�б�����ѧʽ�����ʣ���Ŀ�ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| ���� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| 1 | H | |||||||
| 2 | D | E | F | |||||
| 3 | A | B | C | G |
��2��A��B��C��G����Ԫ�ص�����������Ӧˮ������м�����ǿ����NaOH���ѧʽ����ͬ�����������������������Al��OH��3��������ǿ����HClO4��
��3����3���ڸ�Ԫ���У����ڽ���Ԫ����ԭ�Ӱ뾶��С����Al����Ԫ�ط��ţ���ͬ�����۵���͵���Na����ԭ����ǿ����Na��
��4��EԪ�ص��⻯����H2O���ѧʽ�������⻯���ڳ����¸�A���ʷ�����Ӧ�Ļ�ѧ����ʽ��2Na+2H2O=2NaOH+H2����������Һ��pH��7�����������������=����
��5��GԪ�غ�AԪ���γɵĻ������������Ӿ��壮
CrO42-$\stackrel{H+}{��}$Cr2O72-$\stackrel{Fe_{2}+}{��}$Cr3+$\stackrel{OH-}{��}$Cr��OH��3
��֪��ˮ��Cr�ĺ���Ϊ26.0g•L-1������Һ������Ũ��С��10-5mol/Lʱ��Ϊ�����Ѿ�������ȫ�������£�Ksp[Cr��OH��3]=1��10-32���й������в���ȷ���ǣ�������
| A�� | ��������ת����ֻ��һ���漰������ԭ��Ӧ | |
| B�� | �ڶ������ӷ���ʽΪ��Cr2O72-+14H++6Fe2+=2Cr3++7H2O+6Fe3+ | |
| C�� | ����������ˮ��ʹ��ǿ��Ba��OH��2��Ҳ����ʹ�����ˮ | |
| D�� | ��pH��5ʱ����ˮ�и�Ԫ�س�����ȫ |
| A�� | θ��ƽ����Ҫ����������������θ����ࣺOH-+H+�TH2O | |
| B�� | �����ˮ����2H++CaCO3�TCa2++H2O+CO2�� | |
| C�� | ���ڵij���ʯ��ˮ���ʣ�Ca2++2OH-+2CO2�TCa��HCO3��2 | |
| D�� | ϡ��������⣺6H++Fe2O3�T2Fe3++3H2O |
| A�� | ��ȩ�Ľṹ��ʽΪCH3COH | B�� | ����Ľṹ��ʽΪ��CH3CH3 | ||
| C�� | �ǻ��ĵ���ʽΪ[��O��H]- | D�� | �����ӵĽṹʾ��ͼΪ�� |
| A�� | ������ | B�� | ��°� | C�� | ������ | D�� | �Ž��з� |
| A�� | Ũ��ˮ | B�� | ���� | C�� | ϡ���� | D�� | �ռ���Һ |
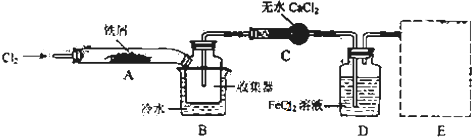

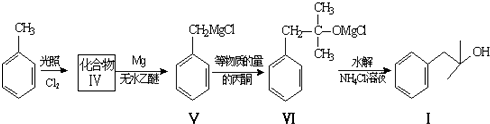
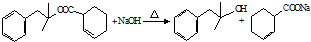 ��
��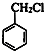 �����ɻ�������ķ�Ӧ����Ϊȡ����Ӧ��
�����ɻ�������ķ�Ӧ����Ϊȡ����Ӧ��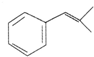 ��
��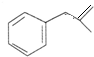 ��
�� Ҳ�ܷ������Ƣ������ķ�Ӧ���ɻ������������Ľṹ��ʽΪ
Ҳ�ܷ������Ƣ������ķ�Ӧ���ɻ������������Ľṹ��ʽΪ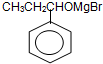 ��
��