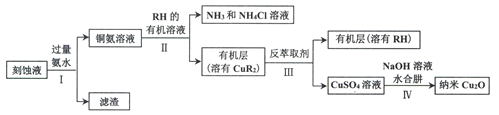
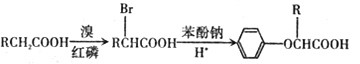��Ŀ����
����Ŀ����֪25 ��ʱ����������ʵĵ���ƽ�ⳣ���������±���
���ữѧʽ | HSCN | CH3COOH | HCN | H2CO3 |
����ƽ�ⳣ�� | 1.3��101 | 1.7��105 | 6.2��1010 | K1=4.3��107 K2=5.6��1011 |
�ش��������⣺
��1��д��̼��ĵ�һ������ƽ�ⳣ������ʽ��K1=_______________________��
��2�������ʵ���Ũ�ȵ�a.CH3COONa��b.NaCN��c.Na2CO3��d.NaHCO3��Һ��pH�ɴ�С��˳��Ϊ________(����ĸ)��
��3�������£�0.1 mol��L1��CH3COOH��Һ��ˮϡ�����У����б���ʽ�����ݱ�����________(�����)
A��[H��] B��[H��]/[CH3COOH]
C��[H��]��[OH] D��[OH]/[H��]
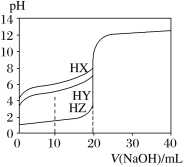
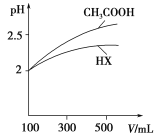
��4�������Ϊ100 mL pH=2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ����HX�ĵ���ƽ�ⳣ��________(����ڡ�����С�ڡ����ڡ�)CH3COOH�ĵ���ƽ�ⳣ����
��5��д������CO2ͨ�����������Һ�е����ӷ���ʽ��_____________________________��
���𰸡�![]() cbdaBDС��CO2��H2O��ClO===HCO3-��HClO
cbdaBD��CO2��H2O��ClO===HCO3-��HClO
��������
��1��̼��ĵ�һ�����뷽��ʽ��![]() �����ݵ��뷽��ʽ��д����ƽ�ⳣ������ʽ����2���������ƽ�ⳣ��ԽС���������ˮ��̶�Խ��ˮ��̶ȣ�CO32-��CN-��HCO3-��CH3COO-��ˮ����Լ��ԣ�ˮ��̶�Խ����Խǿ����3��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c(H+)��С��c(CH3COO-)��С�� Kw������c(OH-)��������4����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȱȴ���С����HX�ȴ����������������ƽ�ⳣ��С����5������HCO3-��HClO��H2CO3������CO2ͨ�����������Һ������̼�����ƺʹ����ᡣ
�����ݵ��뷽��ʽ��д����ƽ�ⳣ������ʽ����2���������ƽ�ⳣ��ԽС���������ˮ��̶�Խ��ˮ��̶ȣ�CO32-��CN-��HCO3-��CH3COO-��ˮ����Լ��ԣ�ˮ��̶�Խ����Խǿ����3��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c(H+)��С��c(CH3COO-)��С�� Kw������c(OH-)��������4����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȱȴ���С����HX�ȴ����������������ƽ�ⳣ��С����5������HCO3-��HClO��H2CO3������CO2ͨ�����������Һ������̼�����ƺʹ����ᡣ
��1��̼��ĵ�һ�����뷽��ʽ��![]() ��̼��ĵ�һ������ƽ�ⳣ������ʽ��K1=
��̼��ĵ�һ������ƽ�ⳣ������ʽ��K1=![]() ����2���������ƽ�ⳣ��ԽС���������ˮ��̶�Խ��ˮ��̶ȣ�CO32-��CN-��HCO3-��CH3COO-��ˮ����Լ��ԣ�ˮ��̶�Խ����Խǿ������pH�ɴ�С��˳��ΪNa2CO3>NaCN>NaHCO3>CH3COONa����3��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С������ƽ�ⳣ��
����2���������ƽ�ⳣ��ԽС���������ˮ��̶�Խ��ˮ��̶ȣ�CO32-��CN-��HCO3-��CH3COO-��ˮ����Լ��ԣ�ˮ��̶�Խ����Խǿ������pH�ɴ�С��˳��ΪNa2CO3>NaCN>NaHCO3>CH3COONa����3��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С������ƽ�ⳣ��![]() ��c��CH3COO-����С������[H��]/[CH3COOH]������ Kw���䣬c(H+)��С������c(OH-)����[OH]/[H��]��������ѡBD����4����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȱȴ���С����HX�ȴ����������������ƽ�ⳣ��С�ڴ����5������HCO3-��HClO��H2CO3������CO2ͨ�����������Һ������̼�����ƺʹ����ᣬ���ӷ���ʽ��CO2��H2O��ClO = HCO3-��HClO��
��c��CH3COO-����С������[H��]/[CH3COOH]������ Kw���䣬c(H+)��С������c(OH-)����[OH]/[H��]��������ѡBD����4����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȱȴ���С����HX�ȴ����������������ƽ�ⳣ��С�ڴ����5������HCO3-��HClO��H2CO3������CO2ͨ�����������Һ������̼�����ƺʹ����ᣬ���ӷ���ʽ��CO2��H2O��ClO = HCO3-��HClO��

����Ŀ���װ�Ǧ�⣨CH3NH3PbI3������ȫ��̬���ѿ�����̫���ܵ�ص�������������CH3NH2��PbI2��HIΪԭ�Ϻϳɣ��ش��������⣺
��1����ȡ�װ��ķ�ӦΪCH3OH��g����NH3��g��![]() CH3NH2��g����H2O��g������H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
CH3NH2��g����H2O��g������H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
���ۼ� | C��O | H��O | N��H | C��N | C��H |
����/kJ��mol��1 | 351 | 463 | 393 | 293 | 414 |
��÷�Ӧ����H��____kJ��mol��1��
��2��������Ӧ������ļ״���ҵ������ˮú���ϳ�CO��g����2H2��g��![]() CH3OH��g������H<0����һ�������£���1 mol CO��2 mol H2ͨ���ܱ������н��з�Ӧ�����ı�ijһ����������¶Ȼ�ѹǿ��ʱ��CH3OH�������������CH3OH���仯������ͼ��ʾ��
CH3OH��g������H<0����һ�������£���1 mol CO��2 mol H2ͨ���ܱ������н��з�Ӧ�����ı�ijһ����������¶Ȼ�ѹǿ��ʱ��CH3OH�������������CH3OH���仯������ͼ��ʾ��
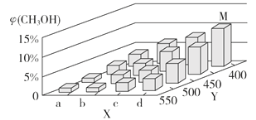
��ƽ��ʱ��M��CH3OH���������Ϊ10%����CO��ת����Ϊ___��
��X����a�����ֵ��b��____ ��������������С������ijͬѧ��Ϊ��ͼ��Y���ʾ�¶ȣ�����Ϊ���жϵ�������_________________��
��3����ҵ�Ͽɲ���CH3OH CO+2H2 ����ȡ�ߴ��ȵ�CO��H2���ҹ�ѧ�߲���������ѧ������ͨ�������ģ�⣬�о������ٻ����������ϼ״�����ķ�Ӧ���̣������������ٴ��������ϵ�������*��ע��
CO+2H2 ����ȡ�ߴ��ȵ�CO��H2���ҹ�ѧ�߲���������ѧ������ͨ�������ģ�⣬�о������ٻ����������ϼ״�����ķ�Ӧ���̣������������ٴ��������ϵ�������*��ע��
�״���CH3OH�����ⷴӦ�ĵ�һ�����̣������ֿ��ܷ�ʽ��
��ʽ A��CH3OH* ��CH3O* ��H* Ea= +103.1kJ��mol-1
��ʽ B��CH3OH* ��CH3* ��OH* Eb= +249.3kJ��mol-1
�ɻ��Eֵ�Ʋ⣬�״��ѽ������Ҫ�����ķ�ʽӦΪ___����A��B����
��ͼΪ�����ģ��ĸ�����Ӧ�������仯ʾ��ͼ��
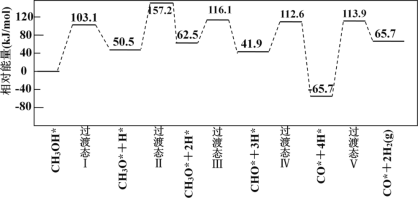
�������У��������IJ���Ļ�ѧ����ʽΪ________��
��4��PbI2��������LiI-Al2O3����Ϊ��������﮵��أ���ṹʾ��ͼ���£�����ܷ�Ӧ�ɱ�ʾΪ��2Li+PbI2=2LiI+Pb����b���ϵĵ缫��ӦʽΪ��_____��

��5��CH3NH2�ĵ��뷽��ʽΪCH3NH2+H2O![]() CH3NH3++OH-���볣��Ϊkb����֪������pkb=-lgkb=3.4����������CH3NH2��Һ�μ�ϡ������c��CH3NH2��=c��CH3NH3+��ʱ����ҺpH=______��
CH3NH3++OH-���볣��Ϊkb����֪������pkb=-lgkb=3.4����������CH3NH2��Һ�μ�ϡ������c��CH3NH2��=c��CH3NH3+��ʱ����ҺpH=______��