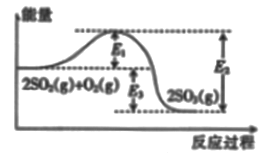
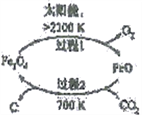
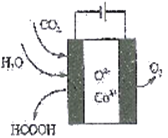
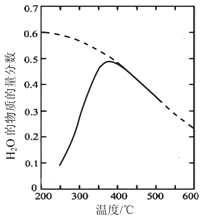
��Ŀ����
����Ŀ������ʵ������淶���ܴﵽʵ��Ŀ�ĵ���
���� | Ŀ�� | |
A | ��ȡ5.0gCuSO4��5H2O����27.0gˮ��,�����ܽ� | ����10%CuSO4��Һ |
B | ����ϡ����ϴ�ӣ�����ˮ��ϴ | ϴ�ӷֽ�KMnO4��O2���Թ� |
C | �ò�����պȡ��Һ�����ڸ����pH��ֽ�ϣ�Ƭ�̺������ɫ���Ƚϲ����� | �ⶨ0.05mol.L-1NaClO��Һ��pH |
D | ���ֵ�����ձ��У��ձ��ڷ�һʢ����ˮ����ƿ����ʯ�������ձ����ȣ�Ȼ���ռ���ƿ��ڵĹ��� | �ᴿ����NH4Cl�Ĵֵ� |
A. A B. B C. C D. D
���𰸡�A
��������A.5.0gCuSO4��5H2O�к�CuSO43.2g������27.0gˮ��,�����ܽ�õ�32gCuSO4��Һ�����ʵ���������Ϊ��![]() ��100��=10��,��A��ȷ��B.ϴ�ӷֽ�KMnO4��O2���Թ�,�Թ��ڱڸ���MnO2��Ҫ��Ũ���Ტ����ϴ�ӣ�����ˮϴ�ӣ���B����C.�ⶨ0.05mol.L-1NaClO��Һ��pH������NaClO����ǿ�����ԣ���ʹ��ֽ��ɫ���ʲ�����pH��ֽ����pH��C����D.�ᴿ����NH4Cl�Ĵֵ�,��NH4Cl���ȷֽ����ɰ������Ȼ��⣬��ȴ���ֽ�ϳ�NH4Cl���ʲ����ü��ȵķ������룬D������ˣ�������ȷ��ΪA��
��100��=10��,��A��ȷ��B.ϴ�ӷֽ�KMnO4��O2���Թ�,�Թ��ڱڸ���MnO2��Ҫ��Ũ���Ტ����ϴ�ӣ�����ˮϴ�ӣ���B����C.�ⶨ0.05mol.L-1NaClO��Һ��pH������NaClO����ǿ�����ԣ���ʹ��ֽ��ɫ���ʲ�����pH��ֽ����pH��C����D.�ᴿ����NH4Cl�Ĵֵ�,��NH4Cl���ȷֽ����ɰ������Ȼ��⣬��ȴ���ֽ�ϳ�NH4Cl���ʲ����ü��ȵķ������룬D������ˣ�������ȷ��ΪA��

��ϰ��ϵ�д�
�����Ŀ