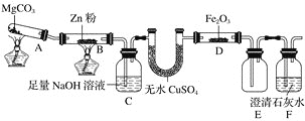
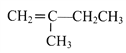��Ŀ����
����Ŀ���й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%-50%������CO2 �ŷ���һ����Ҫ���⡣
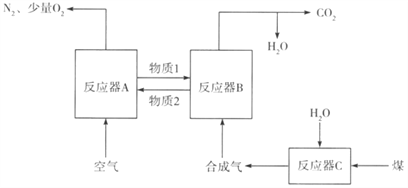
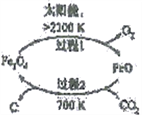
��1����CO2Ϊԭ�ϳ�ȡ̼(C)��̫���ܹ�������ͼ��ʾ��
�ٹ���1��ÿ����1mol FeOת�Ƶ�����Ϊ____________��
�ڹ���2�з�����Ӧ�Ļ�ѧ����ʽΪ________________________________��
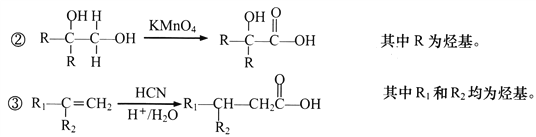
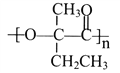
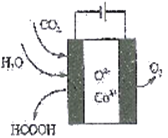
��2��CO2�������ܻ�����������£�ת��Ϊ���ȼ�ϡ������ᡣ�乤��ԭ������ͼ��ʾ��д�����ɼ���ĵ缫��Ӧʽ��____________��
��3������Ա����������CO2�����з�Ӧ��������ʵ�ֿռ�վ��O2��ѭ�����á�
Sabatier��Ӧ��CO2(g)+4H2(g)![]() CH4(g)+2H2O(g)
CH4(g)+2H2O(g)
ˮ��ⷴӦ��2H2O(l)![]() 2H2(g)+O2(g)
2H2(g)+O2(g)
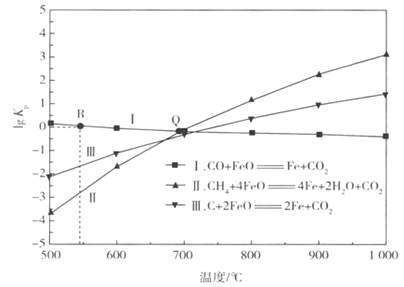
��ԭ������n(CO2)��n(H2)=1��4 �����ܱ������з���Sabatier��Ӧ�����H2O(g)�����ʵ����������¶ȵĹ�ϵ����ͼ��ʾ(���߱�ʾƽ������)��
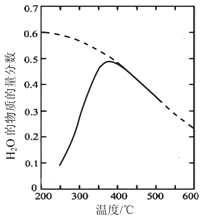
���¶ȹ�����;������ڸ÷�Ӧ�Ľ��У�ԭ����____________________________________��
��200��ﵽƽ��ʱ��ϵ����ѹǿΪp���÷�Ӧƽ�ⳣ���ļ���ʽΪ________________�����ػ�����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ���������
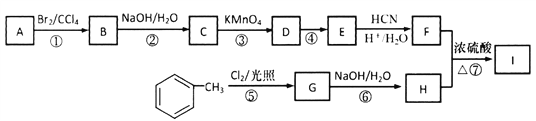
��4��һ���µ�ѭ�����÷�������Bosch��ӦCO2(g)+2H2(g)![]() C(s)+2H2O(g)����Sabatier��Ӧ��
C(s)+2H2O(g)����Sabatier��Ӧ��
����֪CO2(g)��H2O(g)�������ʷֱ�Ϊ-394 kJ��mo1-l��-242 kJ��mo1-l��Bosch��Ӧ�Ħ�H=__________ kJ��mo1-l����������ָһ���������ɶ�Ӧ��������1mol ������ʱ�ķ�Ӧ�ȣ�
��һ��������Bosch��Ӧ�����ڸ����²���������ԭ����______________________________��
���·������ŵ���_________________________��
���𰸡� 2/3NA 6FeO+CO2![]() C+2Fe3O4 CO2+2e-+H2O==HCOOH+O2- �¶ȹ��ͣ���Ӧ����С���¶ȹ��ߣ���Ӧ���ҽ��еij̶�С
C+2Fe3O4 CO2+2e-+H2O==HCOOH+O2- �¶ȹ��ͣ���Ӧ����С���¶ȹ��ߣ���Ӧ���ҽ��еij̶�С  -90 ��Ӧ�Ļ�ܸ� ��ԭ��������Ϊ100%
-90 ��Ӧ�Ļ�ܸ� ��ԭ��������Ϊ100%
����������1���ٸ���ͼ֪��Fe3O4�ڴ���2300Kʱ�ֽ�ΪFeO��O2����Ӧ����ʽΪ2Fe3O4=6FeO+O2����ÿ����6molFeOת��4mol���ӣ�����1��ÿ����1mol FeOת�Ƶ�����Ϊ 2/3 NA���ڹ���2�з�����Ӧ�Ļ�ѧ����ʽΪ6FeO+CO2![]() C+2Fe3O4����2��CO2�������ܻ�����������£�������̼�õ��ӣ����ɼ���ĵ缫��Ӧʽ��CO2+2e-+H2O==HCOOH+O2-����3�����¶ȹ�����;������ڸ÷�Ӧ�Ľ��У�ԭ�����¶ȹ��ͣ���Ӧ����С���¶ȹ��ߣ���Ӧ���ҽ��еij̶�С����200��ﵽƽ��ʱ��ϵ����ѹǿΪp���迪ʼʱn��CO2��Ϊ1mol��n��H2��=4mol����μӷ�Ӧ��n��CO2��Ϊxmol��
C+2Fe3O4����2��CO2�������ܻ�����������£�������̼�õ��ӣ����ɼ���ĵ缫��Ӧʽ��CO2+2e-+H2O==HCOOH+O2-����3�����¶ȹ�����;������ڸ÷�Ӧ�Ľ��У�ԭ�����¶ȹ��ͣ���Ӧ����С���¶ȹ��ߣ���Ӧ���ҽ��еij̶�С����200��ﵽƽ��ʱ��ϵ����ѹǿΪp���迪ʼʱn��CO2��Ϊ1mol��n��H2��=4mol����μӷ�Ӧ��n��CO2��Ϊxmol��
�÷�Ӧ����ʽCO2��g��+4H2��g��![]() CH4��g��+2H2O��g��
CH4��g��+2H2O��g��
��ʼ��mol�� 1 4 0 0
��Ӧ��mol�� x 4x x 2x
ƽ�⣨mol�� 1-x 4-4x x 2x
ˮ���������ʵ�������=![]() =0.6��x=15/16
=0.6��x=15/16
P��CO2��=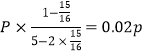 ��P��H2��=
��P��H2��=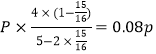 ��P��CH4��=
��P��CH4��= ��P��H2O��=
��P��H2O��=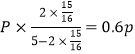 ��ѹǿƽ�ⳣ��K=
��ѹǿƽ�ⳣ��K=![]() =
=![]() ��4����Bosch��Ӧ�ġ�H=2��-242 kJ��mo1-l��-��-394 kJ��mo1-l��=-90 kJ��mo1-l���ڶ��Ѷ�����̼����������������ϸߣ����Ը÷�Ӧ����Ļ�ܽϸߣ���һ��������Bosch��Ӧ�����ڸ����²����������۸÷�Ӧ����Ԫ����ȫת��Ϊˮ����ԭ��������Ϊ100%��
��4����Bosch��Ӧ�ġ�H=2��-242 kJ��mo1-l��-��-394 kJ��mo1-l��=-90 kJ��mo1-l���ڶ��Ѷ�����̼����������������ϸߣ����Ը÷�Ӧ����Ļ�ܽϸߣ���һ��������Bosch��Ӧ�����ڸ����²����������۸÷�Ӧ����Ԫ����ȫת��Ϊˮ����ԭ��������Ϊ100%��

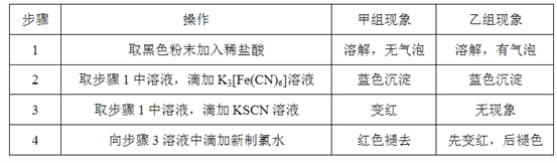
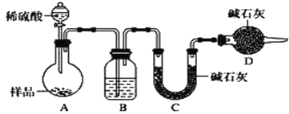
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�����Ŀ������ʵ������淶���ܴﵽʵ��Ŀ�ĵ���
���� | Ŀ�� | |
A | ��ȡ5.0gCuSO4��5H2O����27.0gˮ��,�����ܽ� | ����10%CuSO4��Һ |
B | ����ϡ����ϴ�ӣ�����ˮ��ϴ | ϴ�ӷֽ�KMnO4��O2���Թ� |
C | �ò�����պȡ��Һ�����ڸ����pH��ֽ�ϣ�Ƭ�̺������ɫ���Ƚϲ����� | �ⶨ0.05mol.L-1NaClO��Һ��pH |
D | ���ֵ�����ձ��У��ձ��ڷ�һʢ����ˮ����ƿ����ʯ�������ձ����ȣ�Ȼ���ռ���ƿ��ڵĹ��� | �ᴿ����NH4Cl�Ĵֵ� |
A. A B. B C. C D. D