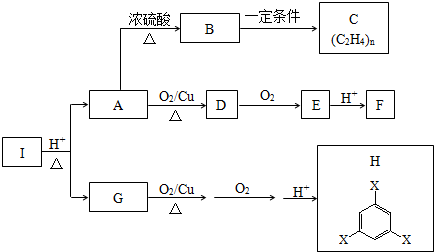
��Ŀ����
17��ij��ѧ��Ȥ̽��С�齫һ����������·������õ���Al��Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ��������������·�ߣ�
�ش��������⣺
��1���ڽ������������ķ�Ӧ�У����������Ե�����ΪNO3-���õ�����1����Ҫ�ɷ�ΪAu��Pt��
��2���ڢڲ���H2O2��Ϊ�˽�һ�ֽ������������������ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��
�������NaOH��Һ��Ŀ����ʹAl3+ת��ΪAlO2-��Fe3+ת��Ϊ�����Ӷ������Ƿֿ���
��3������Һ2��ȡAl2O3������I��ͨ��һ������CO2�������ʽ��Ȼ������ټ��ȣ���Һ�����巴Ӧ�����ӷ���ʽΪ2AlO2-+CO2+3H2O=2Al��OH��3+CO32-��
���� Fe��Al��Au��Pt�Ļ�����м��������Ũ����Ļ�����ܽ⣬���Եõ���Һ1Ϊ���Ρ�������Һ��������1ΪAu��Pt������Һ1�м���˫��ˮ���������ƣ���H2O2�������ǰ�Fe2+����ΪFe3+�����ȹ��˵���Һ2ΪNaAlO2��Һ������2ΪFe��OH��3��NaAlO2��Һ��ͨ�������̼�ٹ��ˡ�ϴ�ӡ����ȷֽ����������Fe��OH��3���ȷֽ����������
��1���ڽ������������ķ�Ӧ�У�����䵱����������������ķ�����֪����1�ijɷݣ�
��2���ڢڲ���H2O2��Ϊ�˽�Fe2+����ΪFe3+����Һ1Ϊ���Ρ�������Һ���������NaOH��Һ��������ƫ��������Һ����������������
��3����Һ2ΪNaAlO2��Һ��ͨ�������̼�ٹ��ˡ�ϴ�ӡ����ȷֽ����������
��� �⣺Fe��Al��Au��Pt�Ļ�����м��������Ũ����Ļ�����ܽ⣬���Եõ���Һ1Ϊ���Ρ�������Һ��������1ΪAu��Pt������Һ1�м���˫��ˮ���������ƣ���H2O2�������ǰ�Fe2+����ΪFe3+�����ȹ��˵���Һ2ΪNaAlO2��Һ������2ΪFe��OH��3��NaAlO2��Һ��ͨ�������̼�ٹ��ˡ�ϴ�ӡ����ȷֽ����������Fe��OH��3���ȷֽ����������
��1���ڽ������������ķ�Ӧ�У�����䵱�����������Ա��������Ե�����ΪNO3-����������ķ�����֪����1�ijɷ�ΪAu��Pt��
�ʴ�Ϊ��NO3-��Au��Pt��
��2���ڢڲ���H2O2��Ϊ�˽�Fe2+����ΪFe3+����Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O����Һ1Ϊ���Ρ�������Һ���������NaOH��Һ��������ƫ��������Һ�������������������Լ������NaOH��Һ��Ŀ����ʹAl3+ת��ΪAlO2-��Fe3+ת��Ϊ�����Ӷ������Ƿֿ���
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��ʹAl3+ת��ΪAlO2-��Fe3+ת��Ϊ�����Ӷ������Ƿֿ���
��3����Һ2ΪNaAlO2��Һ��ͨ�������̼�ٹ��ˡ�ϴ�ӡ����ȷֽ������������Ӧ�����ӷ���ʽΪ2AlO2-+CO2+3H2O=2Al��OH��3+CO32-��
�ʴ�Ϊ��CO2��2AlO2-+CO2+3H2O=2Al��OH��3+CO32-��
���� ���⿼�����ʵķ��롢ʵ����ơ��Լ�ѡ���֪ʶ�㣬��ȷ���ӵ������ǽⱾ��ؼ�����Ŀ�Ѷ��еȣ�

| A�� | ��Һ©��ʹ��ǰҪ������Ƿ�©ˮ | |
| B�� | ��ȡ��Ҫ������ˮ���ұ�ˮ������ʹ���ܽ� | |
| C�� | ��Һʱ���²�Һ��Ӧ���¿��������ϲ�Һ��Ӧ���Ͽڵ��� | |
| D�� | ע���ˮ����ȡ������ת��Һ©�������������ؾ��ã�Ӧ������Һ |
| A�� | �ܵ�������ʾ��ǵ���� | |
| B�� | ���ӷ�Ӧһ��ʹ��Һ���������ӵ�Ũ�ȶ������仯 | |
| C�� | ���ֽ������ӷ�Ӧ����߱��г��������塢�ѵ����������֮һ���������ܷ��� | |
| D�� | ����кͷ�Ӧ���������ӷ���ʽ��H++OH-=H2O��ʾ |
| ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
| A | ����Fe��NO3��2�����Ƿ����������� | ��Fe��NO3��2��Ʒ����ϡH2SO4�μ�KSCN��Һ���۲���Һ�Ƿ��� |
| B | ֤���뱽�������ļ��ױ����� | ��2mL�ױ��м���3������KMnO4��Һ������2mL���м���3������KMnO4��Һ�����۲����� |
| C | �Ƚ�HB��HA������ǿ�� | ȡ�����pH=3��HA��HB������ֱ���������п��Ӧ����ˮ���ռ����壬�۲��ռ����������ĸ��� |
| D | ��֤Fe��OH��3���ܽ��С��Mg��OH��2 | ��FeCl3��Һ����Mg��OH��2����Һ�У����۲����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
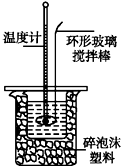
������0.50mol•L-1NaOH��Һ
��1����ʵ����Լ��Ҫ240ml0.50mol•L-1NaOH��Һ����Ӧ����Ͳ��ȡ2.5mol•L-1NaOH��Һ�����Ϊ50.0mL��
��2������0.50mol•L-1NaOH��Һʱ����Ҫʹ�õIJ�����������Ͳ���ձ����������⣬����250mL����ƿ����ͷ�ιܣ�
�ⶨ�к���
ȡ60mL NaOH��Һ��40mL������Һ����ʵ�飬ʵ�����������
| ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | ||
| H2SO4 | NaOH | ƽ��ֵ ������С�����һλ�� | ||
| 1 | 26.3 | 26.0 | 26.1 | 30.1 |
| 2 | 27.0 | 27.3 | 27.2 | 33.3 |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 |
| 4 | 26.5 | 26.2 | 26.3 | 30.4 |
��4������ʵ����ֵ�����57.3kJ•mol-1��ƫ�������ƫ���ԭ�����ad������ĸ����
a��ʵ��װ�ñ��¡�����Ч����
b����ȡ40mL0.50mol•L-1����ʱ���Ӷ���
c�����ᵹ��С�ձ�ʱ�����������ὦ��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��5���ֽ�һ������ϡ����������Һ��ϡ����������Һ��ϡ��ˮ�ֱ��1L 0.50mol/L��ϡ����ǡ����ȫ��Ӧ���䷴Ӧ�ȷֱ�Ϊ��H1����H2����H3�����H1����H2����H3�ɴ�С�Ĺ�ϵΪ��H3����H1����H2��

 ����������д�����з�Ӧ���Ȼ�ѧ����ʽ��
����������д�����з�Ӧ���Ȼ�ѧ����ʽ��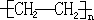 ����Ӧ�����ǼӾ۷�Ӧ��
����Ӧ�����ǼӾ۷�Ӧ�� _��
_��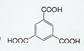 ��
��