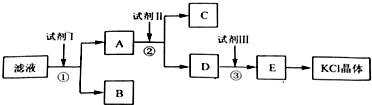
��Ŀ����
9��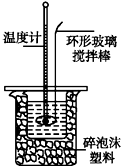 ����������д�����з�Ӧ���Ȼ�ѧ����ʽ��
����������д�����з�Ӧ���Ȼ�ѧ����ʽ����1����������N2��O2��ȫ��Ӧ��ÿ����23g NO2��Ҫ����16.95kJ���������Ȼ�ѧ����ʽΪN2��g��+2O2��g��=2NO2��g����H=+67.8kJ•mol-1
��2����֪��1mol H-H����1mol N-H����1mol N��N���ֱ���Ҫ��������436kJ��391kJ��946kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪN2��g��+3H2��g��?2NH3��g����H=-92kJ•mol-1
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
��1������ĭ���ϵ�������ʲô�����¡����ȣ�����������ʧ
��2��ʵ����ֵ�����57.3kJ•mol-1��ƫ�����ƫ���ԭ���ǣ�����ĸ��˫ѡ��AD��
A��ʵ��װ�ñ��¡�����Ч����
B���ⶨʹ�õ�����ʹ��ǿ��ǿ��
C��һ����NaOH��Һ����ʢ�������С�ձ���
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ�
���� ��1�������Ȼ�ѧ����ʽ����д���������ʵ����������������ȣ�ע�����ʵľۼ�״̬�ͷ�Ӧ�ȵ�λ�����⣻
��2����ѧ��Ӧ�У���ѧ�����������������γ��»�ѧ���ų����������ݷ���ʽ����ֱ����պͷų����������Դ˼��㷴Ӧ�Ȳ�д���Ȼ�ѧ����ʽ��
��1�������к��ȵ�ʵ��Ĺؼ��Ǿ����ܼ�������ɢʧ���н��
��2��A��װ�ñ��¡�����Ч�����õ�����ƫС��
B���ⶨ�к���ʱ����ʹ��ϡ��ǿ���ǿ����Һ��
C�����ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У���õ�����ƫС������Ӧ��һ���Ե�������������Һ��
D���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ�
��� �⣺��1��������N2��O2��ȫ��Ӧ��ÿ����23g NO2��Ҫ����16.95kJ����������ÿ����92��NO2��Ҫ����67.8kJ���������Ȼ�ѧ����ʽΪ��N2��g��+2O2��g��=2NO2��g����H=+67.8kJ•mol-1��
�ʴ�Ϊ��N2��g��+2O2��g��=2NO2��g����H=+67.8kJ•mol-1��
��2����ӦN2+3H2?2NH3�У�����3molH-H����1mol N��N�������յ�����Ϊ��3��436kJ+946kJ=2254kJ������2mol NH3�����γ�6mol N-H�����ų�������Ϊ��6��391kJ=2346kJ�����յ������٣��ų��������࣬�÷�ӦΪ���ȷ�Ӧ���ų�������Ϊ��2346kJ-2252kJ=92kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-92kJ•mol-1��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-92kJ•mol-1��
��1���к��ȵ�ʵ��Ĺؼ��DZ��£���������ĭ���ϵ������DZ��¡����ȣ���ֹ�кͷ�Ӧʱ������ʧ��
�ʴ�Ϊ�����¡����ȣ�����������ʧ��
��2��A��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����A��ȷ��
B���к��ȵIJⶨ�б���ʹ��ϡ��ǿ���ǿ����Һ������ʹ����������������Ӱ��ⶨ��������Ը���������������Ӱ��ⶨ�������B����
C������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У��������������ɢʧ�����Ըò�����������C����
D���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ������¶ȼ��ϻ����������ƣ��������������ᷴӦ���ȣ������������ʼ�¶�ƫ�ߣ���ô������¶��������ͱ�ʵ��ҪС����D��ȷ��
��ѡ��AD��
���� ���⿼���Ȼ�ѧ����ʽ�Լ���Ӧ�ȵIJ�������Ŀ�Ѷȴ�ע�������к��ȵĸ�������Ȼ�ѧ����ʽ����д�����Լ��ⶨ��Ӧ�ȵ��������⣮

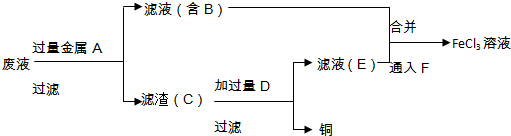
| A�� | Z2+2M2+�T2M3++2Z-�����Խ��� | |
| B�� | ZԪ���ڢ٢۷�Ӧ�з�����ԭ��Ӧ | |
| C�� | �����ӻ�ԭ����ǿ������˳����X2+��Z-��R-��M2+ | |
| D�� | ��������������ǿ������˳����XO4-��Z2��R2��M3+ |
| A�� | ԭ�Ӿ����п��ܴ��ڷǼ��Թ��ۼ� | |
| B�� | ����������ܱȷ��Ӿ����۵�� | |
| C�� | �ɱ�����ʱ�������ڹ��ۼ����ܻᷢ������ | |
| D�� | ����Ԫ�غͷǽ���Ԫ���γɵĻ�������������ӻ����� |

 ��
��