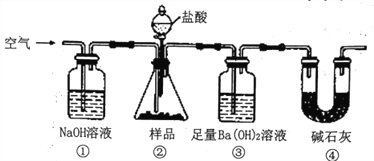
��Ŀ����
����Ŀ���л���A(C6H12O2)���й���ζ��������ʳƷ�����������������Ȼ�ͺϳ���֬���ܼ���

��֪���� D��E������ͬ�����ţ�E����Է���������D��
�� E���Ӻ���֧����
�� F�ǿ���ʹ������Ȼ�̼��Һ��ɫ������
(1) B�Ļ�ѧ����Ϊ____________��D�Ľṹ��ʽ_____________��
(2) C��F�����������Ĺ����ŵ������ֱ���___________��______________��
(3) д���л���B����C�Ļ�ѧ��Ӧ����ʽ��___________________����Ӧ������________��
(4) д���л���B��E��Ӧ����A�Ļ�ѧ��Ӧ����ʽ��_______________________����Ӧ������________��
(5) E��ͬ���칹������ͬʱ�������������Ĺ���________��(���������칹) ����д������һ�ַ���������ͬ���칹��ṹ��ʽ__________________��
������Na��Ӧ�� ���ܷ���������Ӧ��
���𰸡��Ҵ� CH3COOH ȩ�� ̼̼˫�� 2CH3CH2OH��O2 ![]() 2CH3CHO+2H2O ������Ӧ CH3CH(CH3)COOH+CH3CH2OH
2CH3CHO+2H2O ������Ӧ CH3CH(CH3)COOH+CH3CH2OH![]() CH3CH(CH3)COOCH2CH3+H2O ������Ӧ(��ȡ����Ӧ) 5 HOCH2CH2CH2CHO
CH3CH(CH3)COOCH2CH3+H2O ������Ӧ(��ȡ����Ӧ) 5 HOCH2CH2CH2CHO
��������
�л���A(C6H12O2)���й���ζ��������ʳƷ���������AΪ����B��E����������Ӧ����A����B��EΪ�����ᣬB�ܹ�����������Ӧ����C��C�ܹ���������������Ӧ����D����BΪ����DΪ�D��E������ͬ�����ţ�E����Է���������D����E�к����ĸ�̼ԭ�ӣ�B�к���2��̼ԭ�ӣ�E���Ӻ���֧�������BΪ�Ҵ���EΪ(CH3)2CHCOOH����AΪ(CH3)2CHCOOCH2CH3��CΪ��ȩ��DΪ����Ҵ���Ũ�������ʱ������ˮ��Ӧ����F��F�ǿ���ʹ������Ȼ�̼��Һ��ɫ��������FΪ��ϩ���ݴ˷������
��������������AΪ(CH3)2CHCOOCH2CH3��BΪ�Ҵ���CΪ��ȩ��DΪ���ᣬEΪ(CH3)2CHCOOH��FΪ��ϩ��
(1) BΪ�Ҵ���DΪ���ᣬ�ṹ��ʽΪCH3COOH���ʴ�Ϊ���Ҵ���CH3COOH��
(2) CΪ��ȩ��FΪ��ϩ�������Ĺ����ŷֱ���ȩ����̼̼˫�����ʴ�Ϊ��ȩ����̼̼˫����
(3) �л���B����C�Ļ�ѧ��Ӧ����ʽΪ2CH3CH2OH��O2 ![]() 2CH3CHO+2H2O���÷�Ӧ����������Ӧ���ʴ�Ϊ��2CH3CH2OH��O2
2CH3CHO+2H2O���÷�Ӧ����������Ӧ���ʴ�Ϊ��2CH3CH2OH��O2 ![]() 2CH3CHO+2H2O��������Ӧ��
2CH3CHO+2H2O��������Ӧ��
(4) �л���B��E��Ӧ����A�Ļ�ѧ��Ӧ����ʽCH3CH(CH3)COOH+CH3CH2OH![]() CH3CH(CH3)COOCH2CH3+H2O���÷�ӦΪ������Ӧ��Ҳ��ȡ����Ӧ���ʴ�Ϊ��CH3CH(CH3)COOH+CH3CH2OH
CH3CH(CH3)COOCH2CH3+H2O���÷�ӦΪ������Ӧ��Ҳ��ȡ����Ӧ���ʴ�Ϊ��CH3CH(CH3)COOH+CH3CH2OH![]() CH3CH(CH3)COOCH2CH3+H2O��������Ӧ��ȡ����Ӧ��
CH3CH(CH3)COOCH2CH3+H2O��������Ӧ��ȡ����Ӧ��
(5) EΪ(CH3)2CHCOOH��E��ͬ���칹������ͬʱ��������������������Na��Ӧ��˵���ṹ�к����ǻ����Ȼ������ܷ���������Ӧ��˵���ṹ�к���ȩ�������E��ͬ���칹���к���ȩ�����ǻ�����ȥȩ��������3��̼ԭ�ӣ������������У�ȩ��������1��̼ԭ���ϣ��ǻ���3�����ӷ�ʽ��ȩ��������2��̼ԭ���ϣ��ǻ���2�����ӷ�ʽ����5��ͬ���칹�壬��HOCH2CH2CH2CHO��CH3CHOHCH2CHO�ȣ��ʴ�Ϊ��5��HOCH2CH2CH2CHO(��CH3CHOHCH2CHO��)��

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�����Ŀ��X��Y��������������Z��Һ�й�����ͼ��װ�ã�ʵ���е�����ָ�뷢��ƫת��ͬʱX����֣�Y����ϸ����X��Y��Z������( )
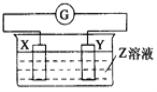
ѡ�� | X | Y | Z |
A | Zn | Cu | ϡ���� |
B | Cu | Zn | ϡ���� |
C | Cu | Ag | ����ͭ��Һ |
D | Ag | Zn | ��������Һ |
A.AB. BC. CD. D