��Ŀ����
�������п��淴Ӧ��A(g)��B(g)  xC(g)���ڲ�ͬ������������C�ڷ�Ӧ������е���������(C%)�ͷ�Ӧʱ��(t)�Ĺ�ϵ����ͼ��
xC(g)���ڲ�ͬ������������C�ڷ�Ӧ������е���������(C%)�ͷ�Ӧʱ��(t)�Ĺ�ϵ����ͼ��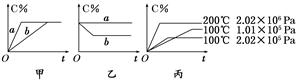
�����ͼ��ش��������⣺
(1)����ͼ���������߷ֱ��ʾ�д������������������ ���߱�ʾ����ʱ�����(����ĸ����ͬ)��
(2)����ͼ��ʾ��Ӧ�ﵽƽ���ֱ��ں��º�ѹ�����ºͺ��º�����������ƽ���������г������(�뷴Ӧ��ϵ����һ���ʾ�����Ӧ)������������� ���߱�ʾ���º��ݵ������
(3)���ݱ�ͼ�����жϸÿ��淴Ӧ������Ӧ�� �ȷ�Ӧ(������š�)��
(4)��ѧ������x��ֵ (��ȡֵ��Χ)���жϵ������� ��
(1)b (2)a (3)��
(4)����2(��>2���3) �����¶Ȳ��䣬����ѹǿ��C%��С��˵��ƽ�����淴Ӧ�����ƶ�����x>1��1��2
����

����β���к���CO��NO2���ж����壬��������װβ������װ�ã���ʹ�ж��������Ӧת��Ϊ�����塣����β����CO��H2O(g)��һ�������¿��Է�����Ӧ��
CO(g)��H2O(g) CO2(g)��H2(g)��H��0��820 ��ʱ�ڼס��ҡ������������ܱ������У���ʼʱ�����±�����Ͷ�ϣ��ﵽƽ��״̬��K��1.0��
CO2(g)��H2(g)��H��0��820 ��ʱ�ڼס��ҡ������������ܱ������У���ʼʱ�����±�����Ͷ�ϣ��ﵽƽ��״̬��K��1.0��
| ��ʼ���ʵ��� | �� | �� | �� |
| n(H2O)/mol | 0.10 | 0.20 | 0.20 |
| n(CO)/mol | 0.10 | 0.10 | 0.20 |
��1���÷�Ӧ��ƽ�ⳣ������ʽΪ ��
��2��ƽ��ʱ����������CO��ת������ ���Ƚ�����������CO��ת���ʣ��� �ף��� ��(�>������������<��)��
��3���������У���Ҫͨ���ı��¶ȣ�ʹCO��ƽ��ת�����������¶���Ҫ���Ͳ��ܴﵽ�����º��ƽ�ⳣ��K (���������С�����䡱)��
��֪NO2��N2O4�����ת����2NO2(g)  N2O4(g)(����ӦΪ���ȷ�Ӧ)���ֽ�һ��
N2O4(g)(����ӦΪ���ȷ�Ӧ)���ֽ�һ��
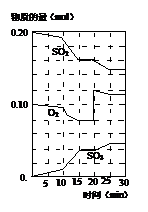
��NO2��N2O4�Ļ������ͨ��һ���Ϊ1 L�ĺ����ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��ʾ���ش��������⣺
(1)ͼ�й�����������X��Y����������________��ʾNO2Ũ����ʱ��ı仯��a��b��c��d�ĸ����У���ʾ��ѧ��Ӧ����ƽ��״̬�ĵ���________��
(2)ǰ10 min����NO2��ʾ�Ļ�ѧ��Ӧ����v(NO2)��________mol/(L��min)����Ӧ������25 minʱ�����߷����仯��ԭ����________��
(3)��Ҫ�ﵽ�������ͬ�Ļ�ѧƽ��״̬����25 minʱ�����Բ�ȡ�Ĵ�ʩ��________��
| A��������� | B����������� |
| C�������¶� | D������һ������N2O4 |
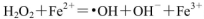
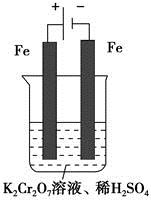
 ����Fe2���ڴ˹����������������______________��������336mL O2����״����ʱ����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ_______mol��
����Fe2���ڴ˹����������������______________��������336mL O2����״����ʱ����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ_______mol��


 .
. CO(g) + H2O(g)��
CO(g) + H2O(g)�� B(g) +C(g)���ݻ�Ϊ1.0L���ܱ������н��У�A�ij�ʼŨ��Ϊ0.050mol/L���¶�T1��T2��A��Ũ����ʱ���ϵ��ͼ��ʾ���ش��������⣺
B(g) +C(g)���ݻ�Ϊ1.0L���ܱ������н��У�A�ij�ʼŨ��Ϊ0.050mol/L���¶�T1��T2��A��Ũ����ʱ���ϵ��ͼ��ʾ���ش��������⣺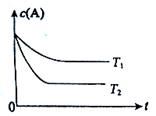
 Cr2O72- (��ɫ)��H2O��
Cr2O72- (��ɫ)��H2O��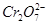 ��
��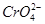 �����õķ��������֡�
�����õķ��������֡�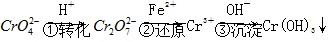 ��
��