��Ŀ����
�����г������ؽ�����Ⱦ���У�����Ǧ���̡������ӡ�������ҵ��ˮ�к��е�Cr2O72-��CrO42-�����õķ���Ϊ��ԭ���������÷��Ĺ�������ΪCrO42-H����ת��Cr2O72-Fe2���ڻ�ԭCr3��OH���۳���Cr(OH)3����
���еڢٲ�����ƽ��2CrO42- (��ɫ)��2H�� Cr2O72- (��ɫ)��H2O��
Cr2O72- (��ɫ)��H2O��
(1)д���ڢٲ���Ӧ��ƽ�ⳣ������ʽ ��
(2)���ڵڢٲ���Ӧ������˵����ȷ���� ��
A��ͨ���ⶨ��Һ��pH�����жϷ�Ӧ�Ƿ��Ѵﵽƽ��״̬
B���÷�ӦΪ������ԭ��Ӧ
C��ǿ���Ի�������Һ����ɫΪ��ɫ
(3)�ڢڲ��У���ԭ0.1 mol Cr2O72-����Ҫ mol��FeSO4��7H2O��
(4)�ڢ۲�������Cr(OH)3�⣬���������ɵij���Ϊ ��
(5)����Һ�д������³����ܽ�ƽ�⣺Cr(OH)3(s)  Cr3��(aq)��3OH��(aq)�������£�Cr(OH)3���ܶȻ�Ksp��10��32����c(Cr3��)����10��5 mol/L����Ϊc(Cr3��)�Ѿ���ȫ�������ֽ��ڢ۲���Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ(��д���������)�� ��
Cr3��(aq)��3OH��(aq)�������£�Cr(OH)3���ܶȻ�Ksp��10��32����c(Cr3��)����10��5 mol/L����Ϊc(Cr3��)�Ѿ���ȫ�������ֽ��ڢ۲���Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ(��д���������)�� ��
(1)K��
(2)AC
(3)0.6
(4)Fe(OH)3
(5)��pH����4ʱ��c(OH��)��10��10 mol/L��c(Cr3��)��10��32/c3(OH��) ��10��2 mol/L��10��5 mol/L�����Cr3��û�г�����ȫ
����

 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д���14�֣��й����������վ������ʾ��������(PM2.5��)Ϊ������������Ӱ�������������������Ⱦ�����ҪΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣��ش��������⣺
��1����PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
| ���� | K+ | Na+ | NH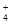 | SO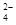 | NO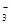 | Cl�� |
| Ũ��/mol?L��1 | 4��10��6 | 6��10��6 | 2��10��5 | 4��10��5 | 3��10��5 | 2��10��5 |
��2�� NOx������β������Ҫ��Ⱦ��֮һ����������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ���£�
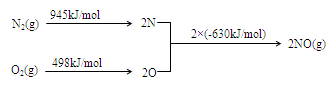
�� N2(g)��O2(g)
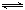 2NO(g)��H�� ��
2NO(g)��H�� ���ڵ�β���п�������ʱ��NOx�ڴ�ת�����б���ԭ��N2�ų���д��NO��CO��ԭ�Ļ�ѧ����ʽ ��
�� �������Ͳ���ȫȼ��ʱ������CO���������밴���з�Ӧ��ȥCO��
2CO(g)��2C(s)��O2(g),��֪�÷�Ӧ�ġ�H��0���������ܷ�ʵ�֣� ��
��3����ѭ�����ղ���������SO2���ͻ�����Ⱦ��ͬʱ�����Ƶ������������������£�
�� �����ӷ���ʽ��ʾ��Ӧ���з����ķ�Ӧ�� ����
�� �û�ѧƽ���ƶ���ԭ����������HI�ֽⷴӦ��ʹ��Ĥ��Ӧ�������H2��Ŀ���� ��
�� ������H2���ϡ������Ͻ���Ϊ��ظ�������(��MH��ʾ����NiO(OH)��Ϊ����������ϣ�KOH��Һ��Ϊ�������Һ�����Ƶø��������������������ء���س�ŵ�ʱ���ܷ�ӦΪ��Ni(OH)2��M
 NiO(OH)��MH����طŵ�ʱ�������缫��ӦʽΪ�� ���� ������ʱ��ȫ��ת��ΪNiO(OH)����������磬����һ���缫����O2��O2��ɢ����һ���缫�����缫��Ӧ�����ģ��Ӷ�������������������ر�ը��
NiO(OH)��MH����طŵ�ʱ�������缫��ӦʽΪ�� ���� ������ʱ��ȫ��ת��ΪNiO(OH)����������磬����һ���缫����O2��O2��ɢ����һ���缫�����缫��Ӧ�����ģ��Ӷ�������������������ر�ը�� ��֪A(g)+B(g) C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
| �¶�/ �� | 700 | 800 | 830 | 1000 | 1200 |
| ƽ�ⳣ�� | 1��7 | 1��1 | 1��0 | 0��6 | 0��4 |
�ش��������⣺
��1���÷�Ӧ��ƽ�ⳣ������ʽK= �� ��H 0���<���� >���� =��)��
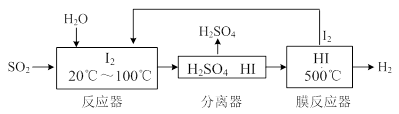
��2��830��ʱ����һ��5 L���ܱ������г���0��20mol��A��0��80mol��B������Ӧ��ʼ6s��A��ƽ����Ӧ����v(A)=0��003 mol��L-1��s-1������6sʱ c(A)= mol��L-1�� C�����ʵ���= mol��
����һ��ʱ���Ӧ�ﵽƽ�⣬���ʱA��ת����= �������ʱ����ܱ��������ٳ���1 mol�����ƽ��ʱA��ת���� �� ����С�����䡱����
��3���жϸ÷�Ӧ�ﵽƽ�������Ϊ ������ȷѡ��ǰ����ĸ)��
A��ѹǿ����ʱ��ı�
B��������ܶȲ���ʱ��ı�
C��c(A)����ʱ��ı�
D����λʱ��������C��D�����ʵ������
��4��1200��ʱ��ӦC(g)+D(g)
 A(g)+B(g)��ƽ�ⳣ����ֵΪ ��
A(g)+B(g)��ƽ�ⳣ����ֵΪ �� ��2 L�ܱ������ڣ�800��ʱ��Ӧ2NO(g)��O2(g)  2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
| ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
| n(NO)(mol) | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
(1)д���÷�Ӧ��ƽ�ⳣ������ʽ��K��__________����֪K300��>K350������÷�Ӧ��________�ȷ�Ӧ��
(2)��ͼ�б�ʾNO2�仯���ߵ���__________����O2��ʾ��0 s��2 s �ڸ÷�Ӧ��ƽ������v��________��
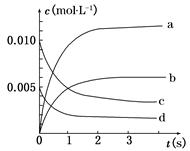
(3)��˵���÷�Ӧ�Ѵﵽƽ��״̬����__________��
a��v(NO2)��2v(O2)
b��������ѹǿ���ֲ���
c��v��(NO)��2v��(O2)
d���������ܶȱ��ֲ���
(4)��ʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ�����__________��
a����ʱ�����NO2����
b���ʵ������¶�
c������O2��Ũ��
d��ѡ���Ч����
�ϳɰ����������Ĵ����������˹��̵���;�����Ի�ѧ��ҵ����Ҳ�������ش�Ӱ�졣�ϳɰ���Ӧ�Ļ�ѧ����ʽΪN2(g)��3H2(g) 2NH3(g)����H����92.2 kJ��mol��1���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����̿��ˮ������Ӧ�Ƶá�
2NH3(g)����H����92.2 kJ��mol��1���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����̿��ˮ������Ӧ�Ƶá�
(1)��һ�ݻ��̶����ܱ�������ע��N2��H2�������壬����������Ӧ����ij�¶��´ﵽƽ��ʱ�������ʵ�Ũ�ȷֱ��ǣ�c(H2)��9.00 mol��L��1��c(N2)��3.00 mol��L��1��c(NH3)��4.00 mol��L��1�����¶��¸÷�Ӧ��ƽ�ⳣ��K��________��
(2)��������ͬ�����и�����1 mol N2��3 mol H2���ڲ�ͬ�����·�Ӧ���ﵽƽ�⣬�������������ʱ��仯��������ͼ��ʾ������˵������ȷ����________(����ĸ)��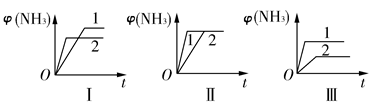
| A��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��p2>p1 |
| B��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��p1>p2 |
| C��ͼ������Dz�ͬ�¶ȶԷ�Ӧ��Ӱ�죬��T1>T2 |
| D��ͼ�������ͬ��ͬѹ�²�ͬ�����Է�Ӧ��Ӱ�죬�Ҵ�������1>2 |
��H����131.3 kJ��mol��1����S����133.7J��K��1 ��mol��1
�÷�Ӧ�ڳ������ܷ��Է����У�________(��ܡ����ܡ�)��
 CH3OH(g)����ҵ��������CO����ȼ�ϼ״���
CH3OH(g)����ҵ��������CO����ȼ�ϼ״��� ��
�� 
 �������____________��
�������____________�� xC(g)���ڲ�ͬ������������C�ڷ�Ӧ������е���������(C%)�ͷ�Ӧʱ��(t)�Ĺ�ϵ����ͼ��
xC(g)���ڲ�ͬ������������C�ڷ�Ӧ������е���������(C%)�ͷ�Ӧʱ��(t)�Ĺ�ϵ����ͼ��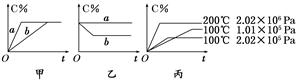
 3C(g)����֪����1molA��3molB�Ҵﵽƽ���������a molC����
3C(g)����֪����1molA��3molB�Ҵﵽƽ���������a molC���� C(g)��D(g)����H��0����ش��������⣺
C(g)��D(g)����H��0����ش��������⣺