��Ŀ����
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�ء�
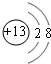
��1��M�����ӽṹʾ��ͼΪ_____;Ԫ��T�����ڱ���λ�ڵ�_____�塣
��2��J������ɵĻ����������6��ԭ�ӣ���ṹ��ʽΪ______��
��3��M��T�γɵĻ������ڳ�ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ_____��
��4��L�������̬�⻯���ˮ��Һ�Լ��ԡ�
�������ӹ�ҵ�У���ˮ��Һ������ʴ��H2O2 �����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ______��
��һ�������£����ڹ̶�������ܱ������з����ֽⷴӦ����H>0������ƽ����ı��±��з�Ӧ����x����ƽ����ϵ����x����y�ݼ�����_______��ѡ����ţ���
| ѡ�� | a | b | c | d |
| x | �¶� | �¶� | ����H2�����ʵ��� | ��������ʵ��� |
| y | �����ʵ��� | ƽ�ⳣ��K | ��ת���� | ���������ʵ����ܺ� |
��5����J��R�γɵ�Һ̬������JR20��2mol��O2����ȫȼ�գ�����������̬�����298Kʱ�ų�����215kJ�� �÷�Ӧ���Ȼ�ѧ����ʽΪ________��
���𰸡�
��1��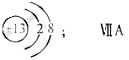
��2��![]()
��3��![]() �������������𰸣�
�������������𰸣�
��4����![]()
��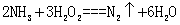
��a��c��a��c
��5��![]()
![]()
����:
��1��JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ������ж�JԪ��Ϊ̼Ԫ�أ�M�ǵؿ��к������Ľ���Ԫ�أ���Ϊ��Ԫ�أ�����J��R���ڱ��е����λ�ÿ����ж�RΪ��Ԫ�أ���TΪ��Ԫ�أ����ڵ������ڵ������塣
��2��J������ɺ���6��ԭ�ӵķ���Ϊ��ϩ����ṹ��ʽΪ![]() ��
��
��3��M��T�γɵĻ�����Ϊ![]() ����ˮ��Ӧ
����ˮ��Ӧ![]() �������Ȼ����������״��
�������Ȼ����������״��
��4���ٰ�ˮ��˫��ˮ����������ԭ��Ӧ![]() ��������Ⱦ�ĵ������ڼ��ڹ���������ܱ������з����ֽⷴӦ��
��������Ⱦ�ĵ������ڼ��ڹ���������ܱ������з����ֽⷴӦ��![]() ����
����![]() ��������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�⳯�������ƶ��������ʵ������٣�����
��������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�⳯�������ƶ��������ʵ������٣�����![]() �����ʵ����������������Ũ�ȣ�ƽ�⳯�淽���ƶ�����ת���ʼ�С��
�����ʵ����������������Ũ�ȣ�ƽ�⳯�淽���ƶ�����ת���ʼ�С��
��5��![]() Ϊ
Ϊ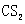 ��ȼ�����ɶ�����̼�Ͷ���������������Ժܿ��д����Ӧ���Ȼ�ѧ����ʽ
��ȼ�����ɶ�����̼�Ͷ���������������Ժܿ��д����Ӧ���Ȼ�ѧ����ʽ![]()
![]() ��
��

 ��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
 Al��OH��3+3HCl
Al��OH��3+3HCl
 J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
 J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�