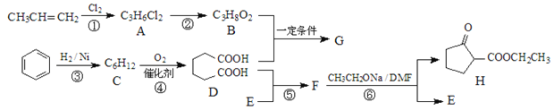
��Ŀ����
����Ŀ����������������Դ�����ˮ�����Ⱦ�ǻ�����������Ҫ�о����⡣
��1����ѧ�ϲ���NH3����NxOy��������������Ⱦ��������Ϊ��ҵ������������Դ��
��֪��![]()
![]()
����NH3����NO���ɵ�������̬ˮ���Ȼ�ѧ����ʽΪ_______________________________��
��2����֪��![]() ����ͬ�¶��£������������зֱ�Ͷ����ͬ���ķ�Ӧ����з�Ӧ����ò�ͬѹǿ��ƽ��������
����ͬ�¶��£������������зֱ�Ͷ����ͬ���ķ�Ӧ����з�Ӧ����ò�ͬѹǿ��ƽ��������![]() �����ʵ���������ͼ��ʾ��
�����ʵ���������ͼ��ʾ��

��M���v��___________Q���v��������>����<����=������
��ͼ��M���ƽ�ⳣ����N���ƽ�ⳣ��___________������������С������ȡ���
��3��ˮ���й�����������![]() ��ʾ���ᵼ��ˮ�帻Ӫ������
��ʾ���ᵼ��ˮ�帻Ӫ������

���ô������Ƴ�ȥ������ԭ����ͼ��ʾ��д���ܷ�Ӧ��ѧ����ʽ____________��
�ڸ÷�Ӧ������¶ȣ��¶ȹ���ʱ����ȥ���ʽ��͵�ԭ����_____________________��
��4����������Ҳ���ü���Һ���ա���NO��![]() ������屻NaOH��Һ��ȫ���գ�ֻ����һ���Σ�����εĻ�ѧʽΪ___________����֪�����£�
������屻NaOH��Һ��ȫ���գ�ֻ����һ���Σ�����εĻ�ѧʽΪ___________����֪�����£�![]() ����Ӧ
����Ӧ![]() ��ƽ�ⳣ������ֵΪ_____________��
��ƽ�ⳣ������ֵΪ_____________��
��5�����÷�Ӧ![]() ��δ��ƽ�������õ���
��δ��ƽ�������õ���![]() �ļ���װ����ͼ��ʾ��
�ļ���װ����ͼ��ʾ��

��a�缫�ϵķ�ӦʽΪ____________________________________________��
�ڳ����£����øõ�ص��0.6L����ʳ��ˮ��һ��ʱ���ñ���ʳ��ˮpH��Ϊ13����������b�缫������B��������Ϊ___________ mL����״�����������������Һ������䣩��
���𰸡�4NH3(g)+6NO(g)�T5N2(g)+6H2O(g) ��H=-1784.4kJmol-1 �� �� 2NH3+3NaClO�TN2+3NaCl+3H2O �¶ȹ��ߣ��ӿ����м����HClO�ķֽ⣬���°���ȥ���ʽ��� NaNO2 5��1010 2NH3-6e-+6OH-=2N2+6H2O 336
��������
(1)���ݸ�˹���ɷ������
(2)��M���Q��ѹǿ��
��M�㰱�������ʵ���������N���Ϸ��ȷ�Ӧ�����жϣ�
(3)�ٸ���ԭ��ͼ��NH3��NaClO�Ƿ�Ӧ������ᡢ���ᡢ��������Ϊ�м����Ȼ��ơ�������ˮ��������ݴ���д��Ӧ�Ļ�ѧ����ʽ���ڸ����¶ȶ��м����HClO��Ӱ��������
(4)NO��NO2������屻NaOH��Һ��ȫ���գ�ֻ����һ���Σ���Ӧ��ֻ��NԪ�ػ��ϼ۷����仯��NO��NԪ�ػ��ϼ�Ϊ+2��NO2��NԪ�ػ��ϼ�Ϊ+4�������ɵ�����NԪ�ػ��ϼ�Ϊ+3�ۣ�����HNO2(aq)+NaOH(aq)NaNO2(ag)+H2O(1)��ƽ�ⳣ������ʽ���ˮ�����ӻ������������㣻
(5)�ٸ���ͼ�е��ӵ��˶������֪��a��Ϊ������Ϊ�����ڼ��������·���������Ӧ���ɵ����ķ�Ӧ���ڸ���2NaCl+2H2O![]() 2NaOH+H2��+Cl2����2e-��һ��ʱ���ñ���ʳ��ˮpH��Ϊ13������Һ��n(OH-)=0.6L��0.1mol/L=0.06mol��b���缫��ӦʽΪ��2NO2+8e-=N2+8OH-����ϵ����غ�������㡣
2NaOH+H2��+Cl2����2e-��һ��ʱ���ñ���ʳ��ˮpH��Ϊ13������Һ��n(OH-)=0.6L��0.1mol/L=0.06mol��b���缫��ӦʽΪ��2NO2+8e-=N2+8OH-����ϵ����غ�������㡣
(1)��2NO(g)=N2(g)+O2(g) ��H=-177kJ/mol����4NH3(g)+3O2(g)�T2N2(g)+6H2O(g) ��H=-1253.4kJ/mol�����ݸ�˹���ɣ�����+����3�ã�4NH3(g)+6NO(g)�T5N2(g)+6H2O(g) ��H=(-1253.4kJ/mol)+(-177kJ/mol)��3=-1784.4kJmol-1���ʴ�Ϊ��4NH3(g)+6NO(g)�T5N2(g)+6H2O(g) ��H=-1784.4kJmol-1��
(2)��M���Q��ѹǿ��ѹǿ����Ӧ���ʼӿ죬��M���v����Q���v�����ʴ�Ϊ������
��M�㰱�������ʵ���������N��÷�Ӧ�Ƿ��ȷ�Ӧ����M����¶ȸߣ������¶�ƽ�������ƶ���ƽ�ⳣ��������M���ƽ�ⳣ����N���ƽ�ⳣ���ʴ�Ϊ����
(3)�ٸ��ݴ������Ƴ�ȥ������ԭ��ͼ��֪��NH3��NaClO�Ƿ�Ӧ������ᡢ���ᡢ��������Ϊ�м����Ȼ��ơ�������ˮ����������Է�Ӧ�Ļ�ѧ����ʽΪ��2NH3+3NaClO�TN2+3NaCl+3H2O���ʴ�Ϊ��2NH3+3NaClO�TN2+3NaCl+3H2O��
���¶ȹ���ʱ���ӿ����м����HClO�ķֽ⣬���°���ȥ���ʽ��ͣ��ʴ�Ϊ���¶ȹ���ʱ���ӿ����м����HClO�ķֽ⣬���°���ȥ���ʽ��ͣ�
(4)NO��NO2������屻NaOH��Һ��ȫ���գ�ֻ����һ���Σ���Ӧ��ֻ��NԪ�ػ��ϼ۷����仯��NO��NԪ�ػ��ϼ�Ϊ+2��NO2��NԪ�ػ��ϼ�Ϊ+4�������ɵ�����NԪ�ػ��ϼ�Ϊ+3�ۣ�Ϊ�������Σ���˻�ѧʽΪNaNO2����ӦHNO2(aq)+NaOH(aq) NaNO2(ag)+H2O(1)��ƽ�ⳣ��K=![]() =
=![]() =
=![]() =
=![]() =5��1010���ʴ�Ϊ��NaNO2��5��1010��
=5��1010���ʴ�Ϊ��NaNO2��5��1010��
(5)�ٸ���ͼ�е��ӵ��˶������֪��a��ʧȥ���ӷ���������Ӧ����Ϊ�����ڼ��������·���������Ӧ���ɵ����ķ�Ӧ���缫��ӦʽΪ��2NH3-6e-+6OH-=2N2+6H2O���ʴ�Ϊ��2NH3-6e-+6OH-=2N2+6H2O��
�ڵ��0.6L����ʳ��ˮ����ⷴӦΪ��2NaCl+2H2O![]() 2NaOH+H2��+Cl2����2e-��һ��ʱ���ñ���ʳ��ˮpH��Ϊ13������Һ��n(OH-)=0.6L��0.1mol/L=0.06mol��ת�Ƶ���Ϊ0.06mol��ͬһ������·ת�Ƶ�����Ŀ��ͬ����B�����ĵ�NO2
2NaOH+H2��+Cl2����2e-��һ��ʱ���ñ���ʳ��ˮpH��Ϊ13������Һ��n(OH-)=0.6L��0.1mol/L=0.06mol��ת�Ƶ���Ϊ0.06mol��ͬһ������·ת�Ƶ�����Ŀ��ͬ����B�����ĵ�NO2
2NO2 + 8e- = N2+8OH-
44.8L 8mol
V0.06mol
��V=![]() =0.336L=336mL���ʴ�Ϊ��336��
=0.336L=336mL���ʴ�Ϊ��336��