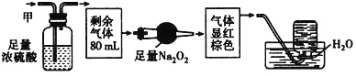
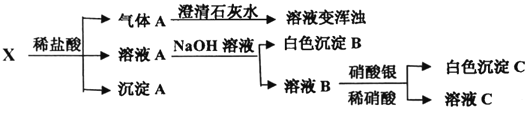
��Ŀ����
����Ŀ��A��B��C��D��E��F��G��Ϊ����������Ԫ�أ���ԭ������������������B�ĵ����ڳ�����Ϊ˫ԭ�ӷ��ӣ�����A�ĵ��ʿ��γɻ�����X��X��ˮ��Һ�ʼ��ԣ�A��Dͬ���壬C��ԭ����������A��Bԭ������֮�ͣ�E�ǵؿ��к�����ߵĽ���Ԫ�أ�FԪ�ص�ԭ�������ȴ�������������ӡ��û�ѧ����ش��������⣺
(1)G��Ԫ�����ڱ��е�λ��Ϊ_______________________��
(2)Ԫ��C��D��E�ļ����ӵİ뾶�ɴ�С��ϵΪ_______________________��
(3)A�ֱ���C��F�γɵ��⻯��е�ϸߵ���_________��ԭ��_______________________��
(4)�õ���ʽ��ʾ������D2C���γɹ���________________________________________��
C��D�����γɻ�����D2C2��D2C2���еĻ�ѧ����_______________________��
���𰸡�����������A�� O2-> Na+> Al3+ H2O H2O���γɷ��Ӽ�������е���� ![]() ���Ӽ����Ǽ��Թ��ۼ�
���Ӽ����Ǽ��Թ��ۼ�
��������
B�ĵ����ڳ�����Ϊ˫ԭ�ӷ��ӣ�����A���γɷ���X��X��ˮ��Һ�ʼ��ԣ��ɴ˿�֪AΪ��Ԫ�أ�BΪ��Ԫ�أ�XΪ������A��Dͬ���壬��D��ԭ���������ڵ�����DΪ��Ԫ�أ�C��ԭ����������A��Bԭ������֮�ͣ���1+7=8����CΪ��Ԫ�أ�E�ǵؿ��к�����ߵĽ���Ԫ�أ���Ϊ��Ԫ�أ�FԪ�ص�ԭ�������ȴ�������������ӣ��ɴ˿�֪FΪ��Ԫ�أ�GΪ��Ԫ�أ��ݴ˷�����
B�ĵ����ڳ�����Ϊ˫ԭ�ӷ��ӣ�����A���γɷ���X��X��ˮ��Һ�ʼ��ԣ��ɴ˿�֪AΪ��Ԫ�أ�BΪ��Ԫ�أ�XΪ������A��Dͬ���壬��D��ԭ���������ڵ�����DΪ��Ԫ�أ�C��ԭ����������A��Bԭ������֮�ͣ���1+7=8����CΪ��Ԫ�أ�E�ǵؿ��к�����ߵĽ���Ԫ�أ���Ϊ��Ԫ�أ�FԪ�ص�ԭ�������ȴ�������������ӣ��ɴ˿�֪FΪ��Ԫ�أ�GΪ��Ԫ�ء�
(1)GΪ��Ԫ�أ���Ԫ�����ڱ��е�λ��Ϊ����������A�壻
(2)������ͬ���Ӳ�ṹ�����ӣ��˵����Խ��뾶ԽС����Ԫ��C��D��E�ļ�����O2-��Na+��Al3+�İ뾶�ɴ�С��ϵΪO2-> Na+> Al3+��
(3)A�ֱ���C��F�γɵ��⻯��H2O��H2S���е�ϸߵ���H2O��ԭ����H2O���γɷ��Ӽ�������е���ߣ�
(4) Na2OΪ���ӻ�����õ���ʽ��ʾNa2O���γɹ���Ϊ��![]() ��
��
C��D�����γɻ�����Na2O2��Na2O2�������Ӻ��������ӹ��ɣ������������д��ڹ��ۼ�����Na2O2�к��еĻ�ѧ�������Ӽ����Ǽ��Թ��ۼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ����û�ѧ����ش��������⣺
�� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� |
��1���ڢۡ���Ԫ���У�ԭ�Ӱ뾶������_____����Ԫ�ط��ţ�����Ԫ�ط���Ϊ_____��
��2����Ԫ�ص�����������Ӧ��ˮ���������⻯���������� M��M �к��еĻ�ѧ��������_____��
��3��д��Ԫ�آٺ͢�ĵ����ڼ��������·�Ӧ���ɵĻ�����ĵ���ʽ��_____��
��4���ۡ��ݡ��ߡ������γɵ����ӣ���뾶��С�����˳����_____�������ӷ��ţ���
��5���١�����Ԫ������������Ӧ��ˮ������������ǿ����_____�������ʻ�ѧʽ���������Ե�����������_____�������ʻ�ѧʽ��,�û�������NaOH ��Һ��Ӧ�����ӷ���ʽΪ_________��
��6���õ���ʽ��ʾԪ�آ�����γɻ�����Ĺ���_____��
����Ŀ��ʵ�� �����ܼ��š� �͡���̼���á��ǻ�ѧ�������о�����Ҫ���⡣��һ����CO2�����״�ȼ�ϵķ�����CO2��g����3H2��g��![]() CH3OH��g����H2O��g����
CH3OH��g����H2O��g����
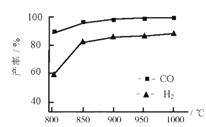
��1��CO2��H2����һ��������ܱ������У������¶���CH3OH�����ʵ�����ʱ��ı仯��ͼ1������I�����Ӧ��ƽ�ⳣ����С��ϵΪK��____K�������������������������

��2��ijʵ���н�6 mol CO2��8 mol H2����2 L���ܱ������У����H2�����ʵ�����ʱ��仯��ͼ2���ߣ��ף���ʾ��
��a������Ӧ����_________�淴Ӧ���ʣ����������������������
0-10min��v��CH3OH�� ��__________mol��L��1��min��1��
�������ı�ijһ�����ٽ���ʵ�飬���H2�����ʵ�����ʱ��仯��ͼ�����ߣ��ң���ʾ�����ߣ��ң���Ӧ�ı��ʵ������������______����ţ���
A���Ӵ��� B������ѹǿ
C�������¶� D������CO2Ũ��
��3��830 �棬��Ӧ��ƽ�ⳣ��K��1����2L���ݷ�Ӧ���з���������Ӧ�����±���A��B��C��D��������ʵ���Ͷ�뷴Ӧ��������������Ӧ������е���_____������ţ���
���� | A | B | C | D |
n��CO2�� | 3 | 1 | 3 | 1 |
n��H2�� | 2 | 2 | 4 | 0 |
n��CH3OH�� | 1 | 2 | 3 | 0.5 |
n��H2O�� | 0 | 2 | 3 | 2 |
��4���о����֣�����ͬ�����»�������һ��ƽ�з�ӦCO2��g����H2��g��![]() CO��g����H2O��g����CO2��H2������ͬ��ʹ�ò�ͬ�Ĵ���ʱ��������CO��CH3OH�����нϴ���죬��ԭ����__________________________ ��
CO��g����H2O��g����CO2��H2������ͬ��ʹ�ò�ͬ�Ĵ���ʱ��������CO��CH3OH�����нϴ���죬��ԭ����__________________________ ��
����Ŀ�����ǵ����Ϻ�����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á���ش��������⣺��ͼ���漰����Ϊ��̬��
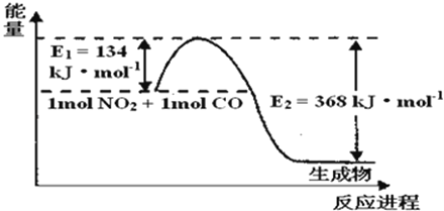
��1����ͼ��1mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ______��
��2����0.5L���ܱ������У�һ�����ĵ����������������»�ѧ��Ӧ��N2��g��+3H2��g��![]() 2NH3��g����H��0���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
2NH3��g����H��0���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
t/�� | 200 | 300 | 400 |
K | K1 | K2 | 0.5 |
������������⣺
���ԱȽ�K1��K2�Ĵ�С��K1______K2����д����������=��������������
�����и�������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������_______���������ĸ����
a .������N2��H2��NH3��Ũ��֮��Ϊ1��3��2 b. ����N2����=3����H2����
c .������ѹǿ���ֲ��� d. ���������ܶȱ��ֲ���
����400��ʱ�������NH3��N2��H2�����ʵ����ֱ�Ϊ1mol��2mol��3molʱ����÷�Ӧ������N2����______����N2��������д��������=��������������