��Ŀ����
��10�֣�Ϊ�˲ⶨʵ���ҳ��ڴ�ŵ�Na2SO3����Ĵ��ȣ�ij��ѧ�С������˶���ʵ�鷽����ȷ��ȡW g������Ʒ�����250mL��Һ���ֳ��������ʵ�飺
���飺��ȡ25.00mL������Һ�����������������ữ��BaCl2��Һ�����ˡ�ϴ�Ӻ���������Ƶó���������Ϊm1 g
���飺��ȡ25.00mL������Һ�����������������ữ��Ba(NO3)2��Һ�����ˡ�ϴ�Ӻ�����������أ�������Ϊm2 g
���飺��ȡ25.00mL������Һ����a mol/L ������KMnO4��Һ���еζ���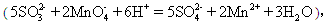 ����KMnO4��Һb mL��
����KMnO4��Һb mL��
��1�� ����250mLNa2SO3��Һʱ�������õ���ʵ�������У��ձ����������ιܺ� ��
��2�� �ڱ���ʵ���еζ�ʱ�Ƿ���Ҫѡ��ָʾ��? (���Ҫ������Ҫ��)���ζ��յ����ɫ�仯�� ��
��3�� �ñ����ʵ�����ݣ�����Na2SO3�Ĵ��� ��
��4�� ʵ���з��֣�����ͬѧ�ⶨ��Na2SO3���ȱȼ���ͱ���ͬѧ�Ľ����Ҫ�͡��Է����������������ԭ�� ��
���飺��ȡ25.00mL������Һ�����������������ữ��BaCl2��Һ�����ˡ�ϴ�Ӻ���������Ƶó���������Ϊm1 g
���飺��ȡ25.00mL������Һ�����������������ữ��Ba(NO3)2��Һ�����ˡ�ϴ�Ӻ�����������أ�������Ϊm2 g
���飺��ȡ25.00mL������Һ����a mol/L ������KMnO4��Һ���еζ���
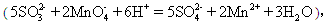 ����KMnO4��Һb mL��
����KMnO4��Һb mL����1�� ����250mLNa2SO3��Һʱ�������õ���ʵ�������У��ձ����������ιܺ� ��
��2�� �ڱ���ʵ���еζ�ʱ�Ƿ���Ҫѡ��ָʾ��? (���Ҫ������Ҫ��)���ζ��յ����ɫ�仯�� ��
��3�� �ñ����ʵ�����ݣ�����Na2SO3�Ĵ��� ��
��4�� ʵ���з��֣�����ͬѧ�ⶨ��Na2SO3���ȱȼ���ͱ���ͬѧ�Ľ����Ҫ�͡��Է����������������ԭ�� ��
��1��������ƽ��250mL������ƿ��2�֣�
��2������Ҫ����ɫ����ɫ����4�֣�
��3�� ��2�֣�
��2�֣�
��4�������ṩH+��Ba(NO3)2�ṩNO3- �γ�ϡHNO3����һ����SO32-������SO42-����Ba2+�������BaSO4��������2�֣�
��2������Ҫ����ɫ����ɫ����4�֣�
��3��
 ��2�֣�
��2�֣���4�������ṩH+��Ba(NO3)2�ṩNO3- �γ�ϡHNO3����һ����SO32-������SO42-����Ba2+�������BaSO4��������2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
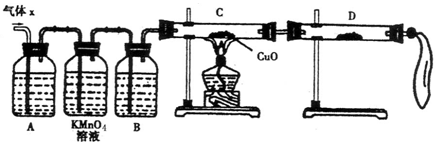
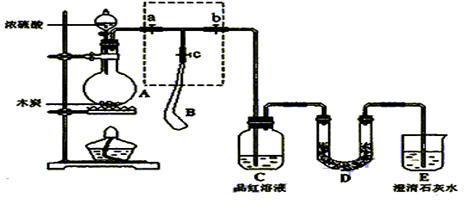 ��ش��������⣺
��ش��������⣺  �ֵ�װ�ã�����ȷ�IJ���
�ֵ�װ�ã�����ȷ�IJ���
 ��Na2SiO3
��Na2SiO3