��Ŀ����
��12�֣�ij��ѧ��ȤС��Ϊ��̽��п��Ũ���ᷴӦ��������ijɷ���������ʵ�飺
��50gп����50mLŨH2SO4�ڼ��������³�ַ�Ӧ��п����ʣ�࣬�ռ���һ����������壬���������������ɱ�״��Ϊ11.2L��
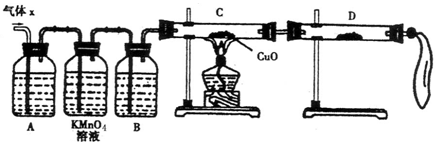
(1)��ѧ��ȤС�����Ƶõ�����X�л��е���Ҫ�������������������������������
�������ʽ�����������ֽ������Ҫԭ���ǣ������ӷ���ʽ��ʾ������������������
��2��ʵ����֤��Ϊ�˼�����Ҫ��������ijɷ֣���ѧ��ȤС���ͬѧ���������ʵ�飬������Xȡ������̽����
��A�м�����Լ�����������������������������������������������������
��B�м�����Լ�������������������
��֤ʵ����X�л����������壬D��Ӧѡ����Լ���������������������ͬʱӦ�۲쵽C�е�ʵ��������������������������������������������
��3�����۷�����
�ٸ�С����ͬѧ���ֻ��Ҫ�ٲ��һ�����ݣ�����ȷ��ȷ�����������ɣ�����Ϊ�������Dz����� ��
A����Ӧ��ʣ��п������Ϊ17.5g
B���ռ������������Ϊ25.8g
C��Ũ��������ʵ���Ũ��Ϊ18.0mol/L
�ڸ������ڢ�����ѡ���ݣ�ͨ������ȷ������X�и��ɷ����ʵ����ֱ�Ϊ����������������������������������������������
��50gп����50mLŨH2SO4�ڼ��������³�ַ�Ӧ��п����ʣ�࣬�ռ���һ����������壬���������������ɱ�״��Ϊ11.2L��

(1)��ѧ��ȤС�����Ƶõ�����X�л��е���Ҫ�������������������������������
�������ʽ�����������ֽ������Ҫԭ���ǣ������ӷ���ʽ��ʾ������������������
��2��ʵ����֤��Ϊ�˼�����Ҫ��������ijɷ֣���ѧ��ȤС���ͬѧ���������ʵ�飬������Xȡ������̽����
��A�м�����Լ�����������������������������������������������������
��B�м�����Լ�������������������
��֤ʵ����X�л����������壬D��Ӧѡ����Լ���������������������ͬʱӦ�۲쵽C�е�ʵ��������������������������������������������
��3�����۷�����
�ٸ�С����ͬѧ���ֻ��Ҫ�ٲ��һ�����ݣ�����ȷ��ȷ�����������ɣ�����Ϊ�������Dz����� ��
A����Ӧ��ʣ��п������Ϊ17.5g
B���ռ������������Ϊ25.8g
C��Ũ��������ʵ���Ũ��Ϊ18.0mol/L
�ڸ������ڢ�����ѡ���ݣ�ͨ������ȷ������X�и��ɷ����ʵ����ֱ�Ϊ����������������������������������������������
��12�֣�
��1��H2 ��Zn+2H+��Zn2++H2����2�֣�
��2����NaOH��Һ(������������)��������SO2���壬ŨH2SO4
����ˮCuSO4����ɫ������
��3����BC����������SO2Ϊ0.4mol��H2Ϊ0.1mol
��1��H2 ��Zn+2H+��Zn2++H2����2�֣�
��2����NaOH��Һ(������������)��������SO2���壬ŨH2SO4
����ˮCuSO4����ɫ������
��3����BC����������SO2Ϊ0.4mol��H2Ϊ0.1mol
п��Ũ���������SO2���壺��Zn��2H2SO4(Ũ)=ZnSO4��SO2����2H2O
��Ũ������Ϊϡ����ʱ������H2����Zn+H2SO4(ϡ)��ZnSO4+H2��
��1���ɼ���ν�����������������
��2��Ϊ�˼��������Ĵ��ڣ������Ŀ����װ�ü�ҩƷ��֪����������ԭCuO����ɫ��ĩ��죩�����ɵ�ˮ����ˮ����ͭ��װ��D��������
���ü�Һ����SO2����ͨ��KMnO4��Һ�Ƿ���ɫ�����鲢�ٴ�����SO2����������Ũ����������壬�õ����﴿����������ͨ��װ��C������ԭCuO��
��3������������SO2��H2�����ʵ����ֱ�Ϊx mol��y mol
�ɢ٢�������ʽ����Ŀ�������ݿ�֪��ȷ���û�����壨SO2��H2������ɣ��Ѿ�֪�����������������״����x+y=11.2/22.4=0.5����ֻҪ֪���ռ��������������Ũ��������ʵ���Ũ�Ⱦ������г�һ��ʽ��������������
64x+2y=25.8��2x+y=0.05��18
��֪����Ӧ��ʣ��п�۵�����ֻ���г�ͬ���ĵ�ʽ������x+y=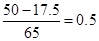 ����δ�������ų�A
����δ�������ų�A
�ʴ�ΪBC
���ս�ã�x=0.4mol y=0.1mol
��Ũ������Ϊϡ����ʱ������H2����Zn+H2SO4(ϡ)��ZnSO4+H2��
��1���ɼ���ν�����������������
��2��Ϊ�˼��������Ĵ��ڣ������Ŀ����װ�ü�ҩƷ��֪����������ԭCuO����ɫ��ĩ��죩�����ɵ�ˮ����ˮ����ͭ��װ��D��������
���ü�Һ����SO2����ͨ��KMnO4��Һ�Ƿ���ɫ�����鲢�ٴ�����SO2����������Ũ����������壬�õ����﴿����������ͨ��װ��C������ԭCuO��
��3������������SO2��H2�����ʵ����ֱ�Ϊx mol��y mol
�ɢ٢�������ʽ����Ŀ�������ݿ�֪��ȷ���û�����壨SO2��H2������ɣ��Ѿ�֪�����������������״����x+y=11.2/22.4=0.5����ֻҪ֪���ռ��������������Ũ��������ʵ���Ũ�Ⱦ������г�һ��ʽ��������������
64x+2y=25.8��2x+y=0.05��18
��֪����Ӧ��ʣ��п�۵�����ֻ���г�ͬ���ĵ�ʽ������x+y=
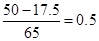 ����δ�������ų�A
����δ�������ų�A�ʴ�ΪBC
���ս�ã�x=0.4mol y=0.1mol

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
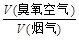 ������Ϊy����ͨ�������г�y��x�Ĺ�ϵʽ��
������Ϊy����ͨ�������г�y��x�Ĺ�ϵʽ��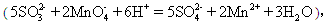 ����KMnO4��Һb mL��
����KMnO4��Һb mL��