��Ŀ����
�����ǻ�ѧ��ҵ�е���Ҫԭ�ϣ���2010���ҹ��ѳ�Ϊȫ�����������ߡ��������Ĺ��ҡ�
(1)18.4mol/L����������0.98���ܶ�1.84g/cm3��Ũ�����dz��õĸ�������������ճ�ʪ�����е�ˮ��������Ũ����Ũ�Ƚ���16 mol/L���ܶ�1.8g/cm3������ʱ����ʧȥ����������
��16 mol/L���������������Ϊ ��������λС������ͬ����
��50mL��������Ϊ0.98��Ũ������Ϊ�����ʱ��������ˮ g��
(2)������������ڸ������������·�Ӧ���õĹ���M 9.920 g��������ϡ���ᷴӦ���ռ�������2.688 L�����㵽��״����������Ϊ3.440 g�������M�ijɷ�Ϊ
��д��ѧʽ����������Ԫ������Ԫ�ص�������Ϊ ��
(3)�������Ṥҵ����ýӴ��������ᣨ��������������������Ϊ0.20����
��Ϊʹ���������ճ�֣���ͨ�����40%�Ŀ����������պ�¯����SO2���������Ϊ ��
�ڽ����е�¯��������������ֱ������Ӵ��ң�����Ϊ1.00m3/s���ӽӴ��ҵ������������Ϊ0.95m3/s��ͬ��ͬѹ�²ⶨ������SO2��ת����Ϊ %��
(4)�Ӵ����������ŷŵ�β�����ð�ˮ���գ�����Ũ���ᴦ�����õ��ϸ�Ũ�ȵ�SO2��ѭ�����ã��ͻ����Ρ�Ϊ�ⶨ������е�Ԫ�ص���������������ͬ��������ηֱ���뵽50.00mL��ͬŨ�ȵ�NaOH��Һ�У���ˮԡ����������ȫ���ݳ������¶�����β��ֽ⣩�������徭�������Ũ����������ȫ���ⶨŨ�������ӵ�������
���ֲⶨ������£�
�������Ϊ10.000 g��20.000 gʱ��Ũ�������ӵ�������ͬ��
�������Ϊ30.000 gʱ��Ũ������������0.680 g��
�������Ϊ40.000 gʱ��Ũ������������䡣
�ټ���û�����е�Ԫ�ص�����������
�ڼ�����������������Һ�����ʵ���Ũ�ȡ�
(1)18.4mol/L����������0.98���ܶ�1.84g/cm3��Ũ�����dz��õĸ�������������ճ�ʪ�����е�ˮ��������Ũ����Ũ�Ƚ���16 mol/L���ܶ�1.8g/cm3������ʱ����ʧȥ����������
��16 mol/L���������������Ϊ ��������λС������ͬ����
��50mL��������Ϊ0.98��Ũ������Ϊ�����ʱ��������ˮ g��
(2)������������ڸ������������·�Ӧ���õĹ���M 9.920 g��������ϡ���ᷴӦ���ռ�������2.688 L�����㵽��״����������Ϊ3.440 g�������M�ijɷ�Ϊ
��д��ѧʽ����������Ԫ������Ԫ�ص�������Ϊ ��
(3)�������Ṥҵ����ýӴ��������ᣨ��������������������Ϊ0.20����
��Ϊʹ���������ճ�֣���ͨ�����40%�Ŀ����������պ�¯����SO2���������Ϊ ��
�ڽ����е�¯��������������ֱ������Ӵ��ң�����Ϊ1.00m3/s���ӽӴ��ҵ������������Ϊ0.95m3/s��ͬ��ͬѹ�²ⶨ������SO2��ת����Ϊ %��
(4)�Ӵ����������ŷŵ�β�����ð�ˮ���գ�����Ũ���ᴦ�����õ��ϸ�Ũ�ȵ�SO2��ѭ�����ã��ͻ����Ρ�Ϊ�ⶨ������е�Ԫ�ص���������������ͬ��������ηֱ���뵽50.00mL��ͬŨ�ȵ�NaOH��Һ�У���ˮԡ����������ȫ���ݳ������¶�����β��ֽ⣩�������徭�������Ũ����������ȫ���ⶨŨ�������ӵ�������
���ֲⶨ������£�
�������Ϊ10.000 g��20.000 gʱ��Ũ�������ӵ�������ͬ��
�������Ϊ30.000 gʱ��Ũ������������0.680 g��
�������Ϊ40.000 gʱ��Ũ������������䡣
�ټ���û�����е�Ԫ�ص�����������
�ڼ�����������������Һ�����ʵ���Ũ�ȡ�
(1)��4�֣��� 0.87����11.63����ÿС��2�֣�
(2)��3�֣�FeS��Fe��1�֣���21��10����2 �֣�
(3)��4�֣��� 0.11���� 92.5%����ÿС��2�֣�
(4)��5�֣�
���裺NaOHΪamol,NH4HSO4Ϊxmol, ��NH4��2SO4Ϊymol��
����
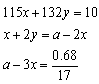
��ã�a=0.232
x=0.064
y=0.02
 ��3�֣�
��3�֣�
�� ��2�֣�
��2�֣�
(2)��3�֣�FeS��Fe��1�֣���21��10����2 �֣�
(3)��4�֣��� 0.11���� 92.5%����ÿС��2�֣�
(4)��5�֣�
���裺NaOHΪamol,NH4HSO4Ϊxmol, ��NH4��2SO4Ϊymol��
����
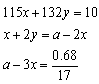
��ã�a=0.232
x=0.064
y=0.02
 ��3�֣�
��3�֣���
 ��2�֣�
��2�֣�(1)��4�֣���

�������������غ�
50ml��1.84g/ml��0.98=16mol/l��(50ml��1.84g/ml+mg)/1.8g/ml��98g��mol-1
m=11.63g
(2)��3�֣�FeS��Fe��1�֣���21��10����2 �֣�
Fe��S
 FeS
FeSFeS��H2SO4=FeSO4��H2S��
Fe��H2SO4=FeSO4��H2��,
n(��)= 2.688 L/22.4l��mol-1=0.12mol
n(H2S)+n(H2)=0.12mol
n(H2S)��34g��mol-1+n(H2)��2 g��mol-1= 3.440 g
n(H2S)=n(FeS)=0.1mol,n(H2)=0.02mol,
m(Fe):m(S)=(9.920g-0.1mol��32g��mol-1):( 0.1mol��32g��mol-1)=21:10
(3)��4�֣��� 0.11���� 92.5%����ÿС��2�֣�
��4FeS2��11O2
 2Fe2O3��8SO2,
2Fe2O3��8SO2,ͨ�����40%�Ŀ����������պ�¯����SO2���������Ϊ
8�£�11��4+11��40%��5+8����100%=8��74��100 %=11%
�� 2 SO2 �� O2
 2SO3 ��V
2SO3 ��V2 1 1
0.1m3/s 0.05m3/s 1.00m3/s�D0.95m3/s
SO2���������1.00m3/s��8��74��100 %="0.108" m3/s��
SO2��ת����Ϊ0.1m3/s��0.108 m3/s��100%=92.5%
(4)��5�֣�
���裺NaOHΪamol,NH4HSO4Ϊxmol, ��NH4��2SO4Ϊymol��
����
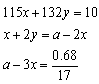
��ã�a=0.232
x=0.064
y=0.02
 ��3�֣�
��3�֣���
 ��2�֣�
��2�֣�
��ϰ��ϵ�д�
 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
�����Ŀ
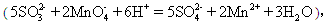 ����KMnO4��Һb mL��
����KMnO4��Һb mL��