��Ŀ����
5��������ǿ�ᣬ��ѧ�ν�������ˮ��Һ�п�����ȫ���룮����ʵ�ǣ�������ˮ�еĵ�һ����������ȫ�ģ��ڶ������벢����ȫ����������Ϊ��H2SO4=H++HSO4-��HSO4-?H++SO42-����ش������й����⣺
��1��K2SO4��Һ�������ԣ�������ԡ��������ԡ��������ԡ�������������SO42-+H2O?HSO4-+OH- �������ӷ���ʽ��ʾ����
��2��NaHSO4��Һ��NaHCO3��Һ��Ӧ�����ӷ���ʽΪHSO4-+HCO3-=H2O+CO2��+SO42-��
��3����25��ʱ��0.10mol/L��NaHSO4��Һ��c��SO42-��=0.03mol/L����HSO4-�ĵ��볣��Ka=0.013��������λ��Ч���֣���0.10mol/L��H2SO4��Һ��c��H+���� 0.13mol/L �����������=����������
���� ��1�������һ����ȫ���룬�ڶ������ֵ��룬˵�����������ˮ�⣬�����������Һ�����������ˮ�����Һ�ʼ��ԣ�
��2���������ƺ�̼��������Һ���ʱ������������Ӻ�̼��������ӷ�Ӧ���ɶ�����̼��ˮ����������ӣ�
��3�����ݵ���ƽ�ⳣ����ʽ���㣻������������������������������ӵ��룮
��� �⣺��1�������һ����ȫ���룬�ڶ������ֵ��룬˵�����������ˮ�⣬�����������Һ�����������ˮ�����Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪSO42-+H2O?HSO4-+OH-��
�ʴ�Ϊ�������ԣ�SO42-+H2O?HSO4-+OH-��
��2���������ƺ�̼��������Һ���ʱ������������Ӻ�̼��������ӷ�Ӧ���ɶ�����̼��ˮ����������ӣ����ӷ���ʽΪHSO4-+HCO3-=H2O+CO2��+SO42-���ʴ�Ϊ��HSO4-+HCO3-=H2O+CO2��+SO42-��
��3��0.10mol•L-1��NaHSO4��Һ��c��SO42-��=0.03mol•L-1����Һ��c��H+��=c��SO42-��=0.03mol•L-1����HSO4-�ĵ��볣��Ka=$\frac{0.03��0.03}{0.10-0.03}$=0.013�������һ���������������������������ӵ��룬������Һ��c��H+����0.13mol•L-1���ʴ�Ϊ��0.013������
���� ���⿼�����������ˮ��Һ�еĵ��룬Ϊ��Ƶ���㣬���ؿ���ѧ����������������ע���������Ϣ�������ע���������������ص㣬Ϊ�״��㣬�ѵ��ǣ�3������㣮

| A�� | �����ǵ�ʵ��ʽ��CH2O | B�� | ��ȩ�Ľṹʽ�� | ||
| C�� | �ǻ��ĵ���ʽ�� | D�� | ��ȩ�ķ���ʽ��CH3CHO |
| A | CH3CH3+Cl2 $\stackrel{����}{��}$CH3CH2Cl+HCl CH2=CH2+HCl��CH3CH2Cl | ��Ϊȡ����Ӧ |
| B | ����֬�õ����� �ɵ��۵õ������� | ��������ˮ�ⷴӦ |
| C | Cl2+2Br?�T2Cl?+Br2 Zn+Cu2+�TZn2++Cu | ��Ϊ���ʱ���ԭ���û���Ӧ |
| D | 2Na2O2+2H2O�T4NaOH+O2�� Cl2+H2O?HCl+HClO | ��Ϊˮ����ԭ����������ԭ��Ӧ |
| A�� | �������м��������ܲ�����������NH4+��SO42-��NO3-��MnO4-һ�������ܹ�������Һ | |
| B�� | ����Һ�к��д���Al3+������Һ�л����ܺ���Mg2+��HCO3-��SO42- | |
| C�� | ����Һ��ˮ���������[H+]=1��10-13mol/L����S2-��SO32-��NH4+���ܴ�������Һ�� | |
| D�� | ����Һ��ʹpH ��ֽ��죬����Һ��I-��Mg2+��NO3-��Cl-���ܴ��� |
| A�� | �����½�pH=3�Ĵ���ϡ�͵�ԭ�����10������Һ��pH=4 | |
| B�� | c�� H+����c��OH-������Һһ�������� | |
| C�� | �����£�pH=6 ����Һһ���������Һ | |
| D�� | ����ˮ���ȣ���KW�����pH���� |
| A�� | ֲ���ƻ����۳����Ʈ�� | B�� | ������������ԭúΪԭ�� | ||
| C�� | ������ˮ�������ŷ� | D�� | �����ŷŵ�β�� |

| A�� | ������̼���⡢����������Ԫ����� | B�� | ���ܷ���������Ӧ | ||
| C�� | ���Dz����͵��л��� | D�� | �������Ǹ߷��ӻ����� |
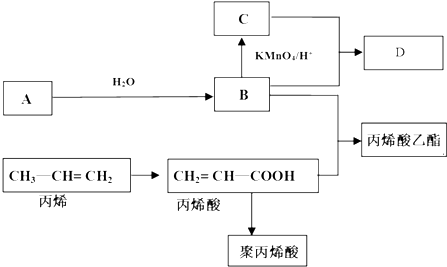
 CH2=CH-COOC2H5+H2O��Ӧ���ͣ�������Ӧ��ȡ����Ӧ
CH2=CH-COOC2H5+H2O��Ӧ���ͣ�������Ӧ��ȡ����Ӧ ��
��