��Ŀ����
����Ҫ��ش����и���
�������� ���� ��NH3������������Һ ��Һ̬�Ȼ��� ��AgCl����ޱ����� �����ǣ���ջش�����ţ���
���������У�1�����ڵ���ʵ��� ����2�����ڷǵ���ʵ��� ��
��3������ǿ����ʵ��� ����4���ܵ������ ��
����д�����з�Ӧ�Ļ�ѧ����ʽ��
����NaHCO3���ɵĻ��Ϸ�Ӧ
�� ��MgCl2�μӵķֽⷴӦ
�� ��Fe2O3�μӵ��û���Ӧ
�� ��HNO3���ɵĸ��ֽⷴӦ
(��).ͬ��ͬѹ�����£�ͬ�����CH4��SO2������֮���� ��ͬ������CH4��SO2�����֮���� ������������ԭ�Ӹ���������ȣ���CH4��SO2������֮���� ��
����������Ƭ��ʯī��������a��b���ַ�ʽ����ʢ��ϡ��������Һ�ͷ�̪��Һ�����Һ�IJ��������У�����һ��ʱ������ȹ۲쵽��Һ���������� ������ţ���
| A����͢� | B����͢����� | C����͢� | D����͢����� |
����12�֣�ÿ��1�֣�����1���ܢݢ��� 2���ڢ���3���ܢ���4���٢�����2NaOH+CO2+H2O=2NaHCO3��NaOH+CO2=NaHCO3 �� MgCl2 Mg+Cl2���� Fe2O3+2Al
Mg+Cl2���� Fe2O3+2Al 2Fe+Al2O3 �� AgNO3+HCl=HNO3+AgCl���������֣�(��).1��4 4��1 3��20 �� ������B
2Fe+Al2O3 �� AgNO3+HCl=HNO3+AgCl���������֣�(��).1��4 4��1 3��20 �� ������B
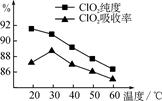
�������������������ʺͷǵ���ʶ��ǻ���������ǰ���½��з��������Ϊ��1���ܢݢޣ� 2���ڢ� ��3���ܢ� ��4���٢� ��������ϵƽʱ��ѧ�Ļ���֪ʶ����ɻ�ѧ����ʽ�����ʾ��(��).ͬ��ͬѹ�����£�ͬ�����CH4��SO2�����ʵ�����ͬ����������֮����Ħ������֮�ȣ���Ϊ1��4 ��ͬ������CH4��SO2�����֮������Ħ�������ķ��ȡ�����������ԭ�Ӹ���������ȣ����趼Ϊ1molת��ΪCH4��SO2������֮����3��20 ��������a�ǵ��أ�bΪԭ��أ���a���൱�ڵ��ˮ���������������ӷŵ磬��Χ�ʼ��ԣ�������������ʴ����̼����Χ��������������������ȹ۲쵽��Һ���������Ǣ�͢�������
���㣺���⿼���˸��������»�ѧ��Ӧ����ʽ����д�͵绯ѧ��Ӧԭ����

 ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д���һ��ɫ����Һ����ȷ���Ƿ����������ӣ�K����Mg2����Al3����Fe2����Ba2����NO3-��SO42-��Cl����I����HCO3-��ȡ����Һ��������ʵ�飺
| ʵ�鲽�� | ʵ������ |
| ��ȡ��������Һ���Ӽ��μ�����Һ | ��Һ���ɫ |
| ��ȡ��������Һ������ͭƬ��Ũ���ᣬ���� | ����ɫ������������������Ա�ɺ���ɫ |
| ��ȡ��������Һ������BaCl2��Һ | �а�ɫ�������� |
| ��ȡ���е��ϲ���Һ������AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����ϡ���� |
| ��ȡ��������Һ������NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ�����������ܽ� |
��1����Һ��һ�����ڵ�������______________����Һ�п϶������ڵ�������________________________��
��2��Ϊ��һ��ȷ���������ӣ�Ӧ�ò����ʵ�鼰��Ӧ���������ӵ�����(��Ϊ��Һ��Ӧ��˵��ʹ���Լ������ƣ�����д��ϸ����)________________________________________��
��3��д��ʵ��������з�Ӧ�����ӷ���ʽ��______________________________��
A��B��C��D�������ʾ�Ϊ����������ɵĿ����Ի����������������ʵ����ӣ����Ӳ����ظ���ϣ��У�
| ������ | Na+��Al3+��Ba2+��NH4+ |
| ������ | Cl����OH����CO32����SO42�� |
�ֱ�ȡ�������ʽ���ʵ�飬ʵ�������¢�B��Һ�ֱ���C��D��ϣ����а�ɫ�������ɢڽ�A��Һ��ε���C��Һ�У��г������ɣ������μ�A��Һʱ����������ֱ����ȫ��ʧ��A��D���ֹ��������������ɣ���������ʹʪ��ĺ�ɫʯ����Һ��������ʯī�缫���B��Һ���������ϲ���һ���д̼�����ζ������
�ش��������⣺
��1��A�����������Ӻ�C���������ӵİ뾶��С____��______�������ӷ��ţ���B��������������________
��2��C��Һ��___�ԣ�����ԡ����ԡ�������ԭ����__________________
�������ӷ���ʽ���ͣ���D�Ļ�ѧʽ��____________
��3����PtΪ�缫���1L0.1mol/LB��ˮ��Һ������·��ͨ��0.1mol����ʱ��
��Һ��pHΪ_______�����������Һ������䣩�������ĵ缫��ӦʽΪ��_____
��4����������������������ͨ��A��Һ����ǡ����ȫ��Ӧʱ������Һ�и�����
Ũ���ɴ�С������˳��Ϊ__________________________