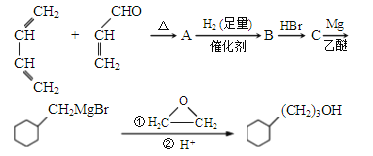
��Ŀ����
����Ŀ��NaCl��Һ�л���Na2SO4��CaCl2��Һ�͵��۽��壬ѡ���ʵ����Լ��ͷ��������ᴿ��NaCl���壮��Ӧ��ʵ�������ͼ��
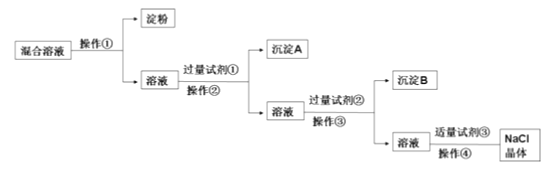
��1��д������ʵ������������Լ����Լ���_________���Լ���________��
��2���ж��Լ����ѹ����ķ����ǣ�____________________��
��3�������������ð�Ĥ���з����ᴿ�������ٵ�ʵ����������_________����ܡ����ܡ�������Ĥ��SO42-________________����ܡ����ܡ�������Ĥ��
��4�������ܵ�������_________��
��5��ʵ�������Ƶõ�NaCl��������480mL1.0mol/L��NaCl��Һ����������ƽ�������Ȼ��ƹ����������_________�����ƹ����õ��IJ�����������Ͳ���ձ�������������ͷ�ι��⣬����_________��
���𰸡�BaCl2 HCl ���ã����ϲ���Һ�еμ������Ȼ�����Һ���ް�ɫ����������˵���Ȼ�����Һ�ѹ��� ���� �� �����ᾧ 29.3g 500mL����ƿ��������
��������
���岻������Ĥ��������Ϊ��������ȥNa2SO4��CaCl2���ɷֱ����BaCl2��Na2CO3����ȥ�����к��е�Ca2+��SO42-�ȿ��������ʵķ������������BaCl2��ȥ����������ӣ��ټ������Na2CO3��ȥ�������ӣ������Լ���ΪBaCl2��������Ϊ���ˣ�����AΪ���ᱵ���Լ���ΪNa2CO3��������Ϊ���ˣ�����BΪ̼��ƺ�̼�ᱵ���Լ���Ϊ���ᣬ��������ɳ�ȥ������Na2CO3����������ᾧ�ɵõ�NaCl���壬�Դ˽����⡣
��1�������Ϸ�����֪�Լ���ΪBaCl2���Լ���ΪHCl��
��2���������ᱵ�Dz�����ˮ�İ�ɫ���������ж��Լ����ѹ����ķ����ǣ����ã����ϲ���Һ�еμ������Ȼ�����Һ��û�а�ɫ����������˵���Ȼ�����Һ�ѹ�����
��3���������Ӻ���Һ���Ӱ뾶�Ƚϴ�������Ĥ������Һ�����ʵ����ӿ�ͨ����Ĥ����˵��۲�������Ĥ��SO42-������Ĥ��
��4������������Һ�õ����壬Ϊ�����ᾧ������
��5��ʵ�������Ƶõ�NaCl��������480mL1.0mol/L��NaCl��Һ��ʵ��Ӧ����500mL����m��NaCl��=0.5L��1mol/L��58.5g/mol=29.25g������������ƽֻ�ܶ�����0.1g������������ƽ����29.3g�����ƹ����õ��IJ�����������Ͳ���ձ�������������ͷ�ι��⣬����500mL ����ƿ����������