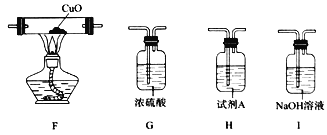
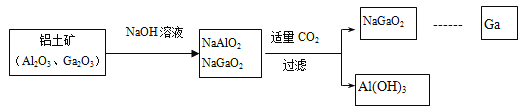
ĚâÄżÄÚČÝ
ˇľĚâÄżˇż¶ĚÖÜĆÚÔŞËؼס˘Ňҡ˘±űˇ˘¶ˇˇ˘ÎěÔÚÔŞËŘÖÜĆÚ±íÖеÄĎŕ¶ÔλÖĂČç±íËůĘľŁ¬ĆäÖжˇËů´¦µÄÖ÷×ĺĐňĘýĘÇĆäÖÜĆÚĐňĘýµÄČý±¶ˇŁ»Ř´đĎÂÁĐÎĘĚ⣺
ŇŇ | ±ű | ¶ˇ | |
Ľ× | Îě |
Ł¨1Ł©ŇŇÔÚÔŞËŘÖÜĆÚ±íÖеÄλÖĂĘÇ___________________ˇŁ
Ł¨2Ł©Ô×Ӱ뾶ŁşÎě______Ľ×Ł¨Ě>ˇ±»ňˇ°<ˇ±Ł©ˇŁ
Ł¨3Ł©ŇŇÓëÎě×éłÉ»ŻşĎÎďµÄµç×ÓʽΪ______Ł¬ĆäÖĐ»ŻŃ§ĽüµÄŔŕĐÍĘÇ_____Ł¨ĚĽ«ĐÔˇ±»ňˇ°·ÇĽ«ĐÔˇ±Ł©ą˛ĽŰĽüˇŁ
Ł¨4Ł©ÔŞËصķǽđĘôĐÔŁşĽ×______±űŁ¨Ě>ˇ±»ňˇ°<ˇ±Ł©Ł¬ĎÂÁĐĘÂʵÄÜ˵Ă÷¸Ă˝áÂ۵ÄĘÇ_______Ł¨Ěî×ÖĸŁ©ˇŁ
AŁ®±űµÄÇ⻯ÎďÎȶ¨Ł¬Ľ×µÄÇ⻯ÎﲻÎȶ¨
BŁ®±űµÄ×î¸ßĽŰŃő»ŻÎď¶ÔÓ¦µÄË®»ŻÎďĘÇÇżËᣬĽ×µÄĘÇČőËá
CŁ®±űµÄ×î¸ßĽŰŃő»ŻÎď¶ÔÓ¦µÄË®»ŻÎďŇ×ČÜÓÚË®Ł¬Ľ×µÄÄŃČÜ
Ł¨5Ł©ą¤ŇµÉĎÖĆȡĽ×Ł¨´ÖĆ·Ł©µÄ»ŻŃ§·˝łĚʽΪ_________________________________ˇŁ
ˇľ´đ°¸ˇż µÚ¶ţÖÜĆÚµÚ˘ôA×ĺ < 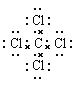 Ľ«ĐÔ < AB SiO2+2C
Ľ«ĐÔ < AB SiO2+2C![]()
![]() Si+2COˇü
Si+2COˇü
ˇľ˝âÎöˇż¸ůľÝ¶ĚÖÜĆÚÔŞËؼס˘Ňҡ˘±űˇ˘¶ˇˇ˘ÎěÔÚÔŞËŘÖÜĆÚ±íÖеÄλÖĂŁ¬żÉÖŞŇҡ˘±űˇ˘¶ˇ´¦ÓÚµÚ¶ţÖÜĆÚŁ¬Ľ×ˇ˘Îě´¦ÓÚµÚČýÖÜĆÚŁ¬¶ˇËů´¦µÄÖ÷×ĺĐňĘýĘÇĆäÖÜĆÚĐňĘýµÄČý±¶Ł¬×îÍâ˛ăµç×ÓĘýÎŞ6Ł¬ąĘ¶ˇÎŞOŁ¬ÔňŇŇÎŞCˇ˘±űÎŞNˇ˘Ľ×ÎŞSiˇ˘ÎěÎŞClˇŁ
Ł¨1Ł©ŇŇÎŞCÔŞËŘŁ¬ÔÚÔŞËŘÖÜĆÚ±íÖеÄλÖĂĘǵڶţÖÜĆÚµÚ˘ôA×壻Ł¨2Ł©Í¬ÖÜĆÚ×Ô×ó¶řÓŇÔ×Ӱ뾶ĽőСŁ¬ąĘÔ×Ӱ뾶ŁşÎěСÓڼף»Ł¨3Ł©ŇŇÓëÎě×éłÉ»ŻşĎÎďÎŞCCl4Ł¬µç×ÓʽΪ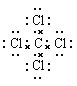 Ł¬ĆäÖĐ»ŻŃ§ĽüµÄŔŕĐÍÎŞŁşĽ«ĐÔą˛ĽŰĽüŁ»Ł¨4Ł©Í¬ÖÜĆÚ×Ô×ó¶řÓŇ·Ç˝đĘôĐÔÔöÇżŁ¬Í¬Ö÷×ĺ×ÔÉ϶řĎ·ǽđĘôĐÔĽőČőŁ¬ÔŞËصķǽđĘôĐÔŁşĽ×ŁĽ±űŁ¬Aˇ˘±űµÄÇ⻯ÎďÎȶ¨Ł¬Ľ×µÄÇ⻯ÎﲻÎȶ¨Ł¬ËµĂ÷±űµÄ·Ç˝đĘôĐÔ¸üÇżŁ¬AŐýČ·Ł»Bˇ˘±űµÄ×î¸ßĽŰŃő»ŻÎď¶ÔÓ¦µÄË®»ŻÎďĘÇÇżËᣬĽ×µÄĘÇČőËᣬ˵Ă÷±űµÄ·Ç˝đĘôĐÔ¸üÇżŁ¬BŐýČ·Ł»Cˇ˘ČÜ˝âĐÔĘôÓÚÎďŔíĐÔÖĘŁ¬˛»ÄܱȽĎÔŞËŘ·Ç˝đĘôĐÔÇżČőŁ¬C´íÎ󣬴đ°¸ŃˇABŁ»Ł¨5Ł©ą¤ŇµÉĎÖƱ¸´ÖąčµÄ·˝łĚʽΪ SiO2 + 2C
Ł¬ĆäÖĐ»ŻŃ§ĽüµÄŔŕĐÍÎŞŁşĽ«ĐÔą˛ĽŰĽüŁ»Ł¨4Ł©Í¬ÖÜĆÚ×Ô×ó¶řÓŇ·Ç˝đĘôĐÔÔöÇżŁ¬Í¬Ö÷×ĺ×ÔÉ϶řĎ·ǽđĘôĐÔĽőČőŁ¬ÔŞËصķǽđĘôĐÔŁşĽ×ŁĽ±űŁ¬Aˇ˘±űµÄÇ⻯ÎďÎȶ¨Ł¬Ľ×µÄÇ⻯ÎﲻÎȶ¨Ł¬ËµĂ÷±űµÄ·Ç˝đĘôĐÔ¸üÇżŁ¬AŐýČ·Ł»Bˇ˘±űµÄ×î¸ßĽŰŃő»ŻÎď¶ÔÓ¦µÄË®»ŻÎďĘÇÇżËᣬĽ×µÄĘÇČőËᣬ˵Ă÷±űµÄ·Ç˝đĘôĐÔ¸üÇżŁ¬BŐýČ·Ł»Cˇ˘ČÜ˝âĐÔĘôÓÚÎďŔíĐÔÖĘŁ¬˛»ÄܱȽĎÔŞËŘ·Ç˝đĘôĐÔÇżČőŁ¬C´íÎ󣬴đ°¸ŃˇABŁ»Ł¨5Ł©ą¤ŇµÉĎÖƱ¸´ÖąčµÄ·˝łĚʽΪ SiO2 + 2C![]() Si + 2CO ˇüˇŁ
Si + 2CO ˇüˇŁ

ˇľĚâÄżˇżZrO2łŁÓĂ×÷ĚմɲÄÁĎŁ¬żÉÓÉďŻÓ˘É°Ł¨Ö÷ŇŞłÉ·ÖÎŞZrSiO4Ł¬Ň˛żÉ±íʾΪZrO2ˇ¤SiO2Ł¬»ąş¬ÉŮÁżFe2O3ˇ˘Al2O3ˇ˘SiO2µČÔÓÖĘŁ©Í¨ąýČçĎ·˝·¨ÖĆȡŁş

ŇŃÖŞŁş˘ŮZrO2ÄÜÓëÉŐĽî·´Ó¦ÉúłÉżÉČÜÓÚË®µÄNa2ZrO3Ł¬Na2ZrO3ÓëËá·´Ó¦ÉúłÉZrO2+ˇŁ
˘Ú˛ż·Ö˝đĘôŔë×ÓÔÚʵŃéĚőĽţĎÂżŞĘĽłÁµíşÍÍęČ«łÁµíµÄpHČçĎÂ±íˇŁ
˝đĘôŔë×Ó | Fe3+ | Al3+ | ZrO2+ |
żŞĘĽłÁµíʱpH | 1.9 | 3.3 | 6.2 |
łÁµíÍęȫʱpH | 3.2 | 5.2 | 8.0 |
Ł¨1Ł©ČŰČÚʱZrSiO4·˘Éú·´Ó¦µÄ»ŻŃ§·˝łĚʽΪ Ł¬ÂËÔüIµÄ»ŻŃ§Ę˝ÎŞ ˇŁ
Ł¨2Ł©ÎŞĘąÂËŇşIµÄÔÓÖĘŔë×ÓłÁµíÍęČ«Ł¬ĐčÓĂ°±Ë®µ÷pH=aŁ¬ÔňaµÄ·¶Î§ĘÇ Ł»ĽĚĐřĽÓ°±Ë®ÖÁpH=bʱŁ¬Ëů·˘Éú·´Ó¦µÄŔë×Ó·˝łĚʽΪ ˇŁ
Ł¨3Ł©ĎňąýÂËIIIËůµĂÂËŇşÖĐĽÓČëCaCO3·ŰÄ©˛˘ĽÓČČŁ¬µĂµ˝Á˝ÖÖĆřĚ塣¸Ă·´Ó¦µÄŔë×Ó·˝łĚʽΪ ˇŁ
Ł¨4Ł©ÎŞµĂµ˝´żľ»µÄZrO2Ł¬ZrŁ¨OHŁ©4ĐčŇŞĎ´µÓŁ¬ĽěŃéZrŁ¨OHŁ©4ĘÇ·ńĎ´µÓ¸Éľ»µÄ·˝·¨ĘÇ ˇŁ
ˇľĚâÄżˇżÄł»ŻŃ§Ń§Ď°Đˇ×éÉčĽĆÁËČçĎ´ӺŁ´ř×ĆÉŐşóµÄşŁ´ř»ŇÖĐĚáȡµâµĄÖʵÄÁ÷łĚŁş

Ł¨1Ł©Čܽ⺣´ř»ŇʱҪĽÓČČÖó·Đ2ˇ«3minµÄÄżµÄĘÇ_________________Ł¬˛Ů×÷aµÄĂűłĆÎŞ ____________ˇŁ
Ł¨2Ł©ĎňËữµÄČÜŇşIÖĐĽÓČëH2O2µÄÄżµÄÎŞ__________________________________ˇŁ
Ł¨3Ł©ŇŃÖŞI2Óë40%µÄNaOHČÜŇş·´Ó¦ÉúłÉµÄŃő»Ż˛úÎďşÍ»ąÔ˛úÎďµÄÎďÖʵÄÁżÖ®±ČÎŞ1:5Ł¬Đ´łö¶ÔÓ¦µÄ»ŻŃ§·˝łĚĘ˝Łş________________________________ˇŁ
Ł¨4Ł©×îşóąýÂ˵õ˝µÄI2ĐčŇŞ˝řĐĐĎ´µÓşÍ¸ÉÔĎÂÁĐĎ´µÓĽÁÖĐ×îÓ¦¸ĂѡÓõÄĘÇ_________Ł¨ĚîѡĎî×ÖĸŁ©ˇŁ A.ČČË® B.ŇŇ´Ľ C.ŔäË® D.¶ţÁň»ŻĚĽ
Ł¨5Ł©ÓĂNa2S2O3µÄ±ę׼ČÜŇş˛â¶¨˛úĆ·µÄ´ż¶ČŁ¬·˘Éú·´Ó¦ŁşI2+2Na2S2O3=Na2S4O6+2NaIˇŁČˇ5.0g˛úĆ·Ł¬ĹäÖĆłÉ100mlČÜŇşˇŁČˇ10.00mlČÜŇşŁ¬ŇÔµí·ŰČÜҺΪָʾĽÁŁ¬ÓĂŨ¶ČÎŞ0.050molˇ¤L-1Na2S2O3µÄ±ę׼ČÜŇş˝řĐеζ¨Ł¬ĎŕąŘĘýľÝĽÇÂĽČçϱíËůĘľˇŁ
±ŕşĹ | 1 | 2 | 3 |
ČÜŇşµÄĚĺ»ý/mL | 10.00 | 10.00 | 10.00 |
ĎűşÄNa2S2O3Ł¬±ę׼ČÜŇşµÄĚĺ»ý/mL | 19.95 | 17.10 | 20.05 |
µÎ¶¨Ę±Ł¬´ďµ˝µÎ¶¨ÖŐµăµÄĎÖĎóĘÇ________________Ł¬µâµĄÖĘÔÚ˛úĆ·ÖеÄÖĘÁż·ÖĘýĘÇ________________Ł¨ÓĂ°Ů·ÖĘý±íĘľŁ¬ÇұŁÁô1λСĘýŁ©ˇŁ