��Ŀ����
����Ŀ�����⻯��(NaAlH4)���л��ϳɵ���Ҫ��ԭ������ϳ���·����ͼ��ʾ��
![]()
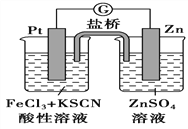
��1�����⻯����ˮ�������ҷ�Ӧ���䷴Ӧ�Ļ�ѧ����ʽΪ__________________________��
��2��AlCl3��NaH��Ӧʱ���轫AlCl3�����л��ܼ����ٽ��õ�����Һ�μӵ�NaH��ĩ�ϣ��˷�Ӧ��NaH��ת���ʽϵ͵�ԭ����__________________________________________��
��3��ʵ����������ͼװ����ȡ��ˮAlCl3��
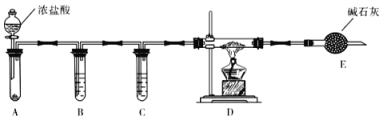
��A����ʢװ���Լ���_______________��
�ڵ�ȼD���ƾ���֮ǰ���ų�װ���еĿ������������____________________________
��4���ı�A��D�е��Լ��Ϳ����ø�װ����ȡNaH����װ���в������������Ƶõ�NaH�п��ܺ��е�����Ϊ____________ ��
��5���������������װ�ã��ⶨ���⻯�ƴֲ�Ʒ(ֻ����NaH����)�Ĵ��ȡ�
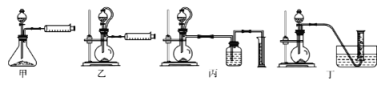
�Ӽ�Լ�ԡ�ȷ�Կ��ǣ������˵�װ����_________(����)����ȡ15.6g��Ʒ��ˮ��ȫ��Ӧ��������ڱ�״���µ����Ϊ22.4L����Ʒ�����⻯�Ƶ�����Ϊ___________��
���𰸡�NaAlH4+2H2O=NaAlO2+4H2�� ��Ӧ���ɵ�NaCl������NaH����,��ֹAlCl3��NaH��Ӧ�Ľ��С���:NaHΪ���ӻ�����,�������л��ܼ�,ʹ��Ӧ�����ԽӴ�������Ӧ KMnO4��KClO3��K2Cr2O7��Ca(ClO)2�� ��Һ©������ʹA�з�����Ӧ,��D�г�������ɫ����ʱ��ȼ�ƾ��� Na2O2 �� 10.8 g
��������
��1�����⻯����ˮ�������ҷ�Ӧ����ƫ�����ƺ�������
��2��NaCl�����ӻ�������л���֮�以�����ܣ����Է�Ӧ���ɵ��Ȼ��Ƴ������⻯�Ʊ��棬��ֹ���Ȼ������⻯�ƽ�һ����Ӧ��
��3���Ȼ������Ʊ��ǽ�����������֮�䷴Ӧ�IJ��Ũ���������أ�����ػ��ظ���أ�֮���������ķ�Ӧ���������������ǽ������ױ��������������ʣ����Ե�ȼD���ƾ���֮ǰ���ų�װ���еĿ�����
��4�������ƺ�����֮�仯�Ͽ��Ե�NaH�����������е��Ȼ���Ҫ��ȥ�������ƺ����������»���������������ʣ�
��5���Ӽ�Լ�ԡ�ȷ�Կ��ǣ��ײ����������ʲ����ȣ������������к��д������������ݷ���ʽ�����ݹ�������������֮���ϵʽ�з���ʽ����������⻯��������
��1�����⻯����ˮ�������ҷ�Ӧ����ƫ�����ƺ��������䷴Ӧ�Ļ�ѧ����ʽΪNaAlH4+2H2O=NaAlO2+4H2�����ʴ�Ϊ��NaAlH4+2H2O=NaAlO2+4H2����
��2��AlCl3��NaH��Ӧʱ���轫AlCl3�����л��ܼ����ٽ��õ�����Һ�μӵ�NaH��ĩ�ϣ������ɵ�NaCl�����ӻ�������л���֮�以�����ܣ����Է�Ӧ���ɵ��Ȼ��Ƴ������⻯�Ʊ��棬��ֹ���Ȼ������⻯�ƽ�һ����Ӧ������NaH��ת���ʽϵͣ��ʴ�Ϊ����Ӧ���ɵ��Ȼ��Ƴ������⻯�Ʊ��棬��ֹ���Ȼ������⻯�ƽ�һ����Ӧ��
��3�����Ȼ������Ʊ��ǽ�����������֮�䷴Ӧ�IJ����װ��AΪ�Ʊ������ķ���װ�ã�Ũ���������أ�����ػ��ظ���أ�֮���������ķ�Ӧ����������������A����ʢװ���Լ�������Ϊ������أ�����ػ��ظ���أ����ʴ�Ϊ��������أ�����ػ��ظ���أ���
����Ϊ�������ױ��������������ʣ����Ե�ȼD���ƾ���֮ǰ���ų�װ���еĿ���������Һ©���Ļ���ʹA�з�����Ӧ����D�г�������ɫ������ʱ��ȼ�ƾ��ƣ��ʴ�Ϊ����Һ©���Ļ���ʹA�з�����Ӧ����D�г�������ɫ������ʱ��ȼ�ƾ��ƣ�
��4�������ƺ�����֮�仯�Ͽ��Ե�NaH�����������е��Ȼ���Ҫ��ȥ�������ƺ����������»���������������ʣ��ʴ�Ϊ��Na2O2��
��5���Ӽ�Լ�ԡ�ȷ�Կ��ǣ��ײ����������ʲ����ȣ������������к��д��������������⼸��װ�����ϴ�ѡ�ң�15.6g��Ʒ��ˮ��Ӧ�Ļ�ѧ����ʽΪ��NaAlH4+2H2O=
NaAlO2+4H2����NaH+H2O=NaOH+H2������n��NaAlH4��=xmol��n��NaH��=ymol���ɷ���ʽ���������ݵ���������ʽ��4x+y=1��54x+24y=15.6������������ʽ�ɵ�x=y=0.2mol����m��NaAlH4��=0.2mol��54g/mol=10.8g���ʴ�Ϊ���ң�10.8g��