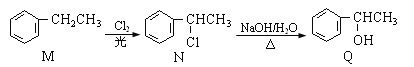��Ŀ����
����Ŀ����ͼ��ʾ2��ʵ��װ�ã��ֱ�ش��������⡣
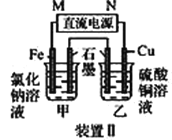
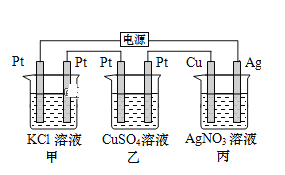
(1)װ�â�Ϊ����������ʴʵ�顣һ��ʱ�������________(� ��������ԭ��); �����ʯī���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽ʯī��������Һ��죬�õ缫��ӦΪ______________________________________��
(2)װ�â��м��ձ�ʢ��100 mL 0.2 mol��L��1��NaCl��Һ�����ձ�ʢ��100 mL 0.5 mol��L��1��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣����ձ��е��뼸�η�̪��Һ���۲쵽��Ƭ�缫�������ȱ�졣��Դ��M��Ϊ________(���������)�������ձ������缫�ĵ缫��ӦΪ____________��ֹͣ��⣬����________�缫�������ӡ�
���𰸡����� O2��2H2O��4e��=4OH�� �� 2H2O+2e-=H2��+2OH- ʯī
��������
��1��������������ʴ��������������Fe2+������������ԭ��Ӧ�������õ����ӱ���ԭ����OH-��
��2����Ӧһ��ʱ���ֹͣͨ�磮����ձ��е��뼸�η�̪���۲쵽���缫�������ȱ�죬˵�������缫������OH-���ӣ��缫��ӦΪ��2H2O+2e-�T2OH-+H2����������ԭ��Ӧ��Ϊ���ص����������ӵ�Դ�ĸ�������M��Ϊ������N��Ϊ������
���ձ��������ͭ��Һ��ʯīΪ�������缫��ӦΪCu2++2e-�TCu���ݴ˷�����
��1��������������ʴ��������������Fe2+������������ԭ��Ӧ�������õ����ӱ���ԭ����OH-���缫����ʽΪO2+4e-+2H2O-�T4OH-��
�ʴ�Ϊ��������O2+4e-+2H2O-�T4OH-��
��2����Ӧһ��ʱ���ֹͣͨ�磮����ձ��е��뼸�η�̪���۲쵽���缫�������ȱ�죬˵�������缫������OH-���ӣ��缫��ӦΪ��2H2O+2e-�T2OH-+H2����������ԭ��Ӧ��Ϊ���ص����������ӵ�Դ�ĸ�������M��Ϊ������N��Ϊ������������ӦΪ2H2O+2e-=H2��+2OH-��
�ʴ�Ϊ������2H2O+2e-=H2��+2OH-��
���ձ��������ͭ��Һ��ʯīΪ�������缫��ӦΪCu2++2e-�TCu�����������ӣ�
�ʴ�Ϊ��ʯī��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���±��г��ˢ١�������Ԫ�������ڱ��е�λ�ã�
�� ���� | ��A | 0 | ||||||
1 | ��A | ��A | ��A | ��A | ��A | ��A | ||
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | ||||
�밴Ҫ��ش��������⡣
(1)Ԫ�آٵ�Ԫ�ط�����_____________��
(2)Ԫ�آڵĵ��ʵ���ʽ��______________��
(3)Ԫ�آݵ�ԭ�ӽṹʾ��ͼ��______________��
(4)������Ԫ���У�λ�ڵ���������ԭ�Ӱ뾶��С����(��Ԫ�ط���)_______��
(5)������Ԫ�ص�����������У����������������Ԫ����(��Ԫ�ط���)_______��
(6)��ʢ��ˮ��С�ձ��м���Ԫ�آ۵ĵ��ʣ�������Ӧ�����ӷ���ʽΪ_______________________����������Ӧ�����Һ���ټ���Ԫ�آܵĵ��ʣ�������Ӧ�Ļ�ѧ����ʽΪ______________________��
����Ŀ��ijʵ��С������ʵ�����ռ�һƿ���﴿������������ʵ��װ�ü�ҩƷ���¡�

��1������NaCl��������________��
��2������������Һ�������������ӷ���ʽΪ________��
��3����֪����ͬ�¶���MnO2�����ᷴӦ��ƽ�ⳣ��
�¶�t/�� | 50 | 80 | 110 |
ƽ�ⳣ��K | 3.104��10-4 | 2.347��10-3 | 1.293��10-2 |
MnO2������ķ�Ӧ��________��������������������������
��4�� ʵ�����4 mol/L����ʱ��û�в�������ʵ������С��ѧ���²�����Ƿ�Ӧ����������ԭ������ǣ�________
�� ��Ӧ�¶ȵͣ�������Ũ�ȵ͡�
Ϊ̽�������Է�Ӧ���ʵ�Ӱ�죬С��ͬѧ��Ʋ��������ʵ�飺
��� | �Լ� | ���� | ���� |
ʵ��1 | 4 mol/L���ᡢMnO2 | ���� | ���������� |
ʵ��2 | 7 mol/LŨ���ᡢMnO2 | ������ | ���������� |
ʵ��3 | 7 mol/LŨ���ᡢMnO2 | ���� | ��������ɫ���� |
�� ��������ʵ���֪MnO2�����ᷴӦ��������������Ϊ________��
�� С��ѧ����һ���²⣺
��������c��H+����СӰ���˷�Ӧ���ʡ�
��������c��Cl���� ��СӰ���˷�Ӧ���ʡ�
���ʵ�鷽��̽����С��ѧ���IJ��롣
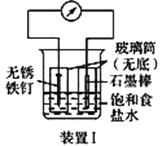
�� Ϊ̽������H+Ũ�ȣ�����ǿ��MnO2�������ԣ�������ǿ��Cl-�Ļ�ԭ�ԣ�С��ͬѧ���������ʵ�顣

ʵ�����Լ�X��________����ͨ��·��ָ�뼸��������ƫת�������Ҳ������еμ�ŨH2SO4��ָ��ƫת����û�б仯��������������еμӵ����ŨH2SO4��ָ������ƫת������Եõ��Ľ�����________��