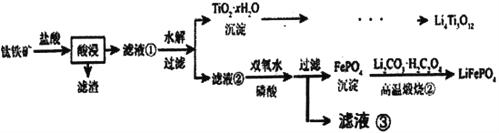
��Ŀ����
����Ŀ����ѧС�������·����ⶨ��������ķ�ˮ�б��ӵĺ�������ˮ�в������Ųⶨ�����ʣ���
������ȷ������KBrO3��������һ�������a mol��L1 KBrO3����Һ��
��ȡv1 mL������Һ���������KBr����HSO4�ữ����Һ��ɫ���ػ�ɫ��
�����������Һ�м���v2 mL��ˮ��
�������������KI��
������b mol��L1 Na2S2O3����Һ�ζ�������Һ��dz��ɫʱ���μ�2�ε�����Һ�������ζ����յ㣬������Na2S2O3��Һv2 mL��
��֪��I2+2Na2S2O3=2NaI+ Na2S4O6
Na2S2O3��Na2S4O6��Һ��ɫ��Ϊ��ɫ
��1������������Һ�õ��IJ����������ձ�������������ͷ�ιܺ�____________��
��2�����з�����Ӧ�����ӷ���ʽ��_______________________________��
��3�����з�����Ӧ�Ļ�ѧ����ʽ��_________________________________��
��4�����м�KIǰ����Һ��ɫ��Ϊ��ɫ��ԭ����______________________________��
��5��KI��KBrO3���ʵ�����ϵΪn��KI����6n��KBrO3��ʱ��KIһ��������������________��
��6�����еζ����յ��������_____________________________��
��7����ˮ�б��ӵĺ���Ϊ___________g��L1������Ħ��������94 g��mol 1����
��8������Br2����____________���ʣ���~���з�Ӧ�����ܱ������н��У��������ɲⶨ���ƫ�ߡ�
���𰸡�����ƿ����Ͳ BrO3- + 5Br- + 6H+ = 3Br2 + 3H2O  �������ɵ�Br2���ˮ�б�����ȫ��Ӧ������Һ��ɫΪ��ɫ��˵����Br2ʣ�࣬ʣ��Br2�����KI��Ӧ����I2�����ú����ζ����������Ӷ���Ӽ��㱽�����ĵ�Br2 ���з�ӦΪKBrO3 + 5KBr + 3H2SO4=3K2SO4+ 3Br2 + 3H2O��֪3n(KBrO3)=n(Br2)������Br2�����뱽�ӷ�Ӧ��ʣ�����ڢ��з�ӦΪBr2+2KI=I2+2KBr����ʣ������ȫ��Ӧ����n(KI)�� 2n(Br2)����֪n(KI)��6n(KBrO3) ���������һ��Na2S2O3����Һʱ����Һ����ɫ��Ϊ��ɫ����30s����ɫ
�������ɵ�Br2���ˮ�б�����ȫ��Ӧ������Һ��ɫΪ��ɫ��˵����Br2ʣ�࣬ʣ��Br2�����KI��Ӧ����I2�����ú����ζ����������Ӷ���Ӽ��㱽�����ĵ�Br2 ���з�ӦΪKBrO3 + 5KBr + 3H2SO4=3K2SO4+ 3Br2 + 3H2O��֪3n(KBrO3)=n(Br2)������Br2�����뱽�ӷ�Ӧ��ʣ�����ڢ��з�ӦΪBr2+2KI=I2+2KBr����ʣ������ȫ��Ӧ����n(KI)�� 2n(Br2)����֪n(KI)��6n(KBrO3) ���������һ��Na2S2O3����Һʱ����Һ����ɫ��Ϊ��ɫ����30s����ɫ ![]() �ӷ�
�ӷ�
��������
���⿼��������ԭ��Ӧ�ζ����ۺ����á��������巴Ӧ�������������ζ��յ������жϣ�����Ƶ�һ��������ֱ��뱽�Ӻ�KI��Ӧ��������ȫ��Ӧ�꣩��������KI��Ӧ���ɵ�I2��Na2S2O3���еζ����������ֱ�Ӳ����KI��Ӧ�����ĵ��壬����������뱽�ӷ�Ӧ���ĵ��壬�����ݱ������巴Ӧ��ϵ�������ˮ�б��ӵ�Ũ�ȡ�
��1��ȷ����KBrO3����������Һ����IJ����������ձ�����Ͳ������������ͷ�ιܣ�һ����������ƿ������ÿ�������ƿ����Ͳ��
��2��KBrO3��Һ�м���KBr��H2SO4����Һ��ɫ���ػ�ɫ��˵������Br2������ȱ����ƽ��֪�����ӷ���ʽΪBrO3- + 5Br- + 6H+ = 3Br2 + 3H2O��
��3�����Ӻ���ˮ��Ӧ�õ���ɫ����2,4,6-���屽�ӣ���ѧ����ʽΪ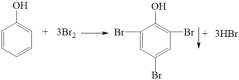 ��
��
��4���ò�������������һ��������ֱ��뱽�Ӻ�KI��Ӧ��ע�����뷴Ӧ��ȫ����һ�������������֪����������KI��Ӧ���ɵ�I2������������ԭ�ζ��������������������KI��Ӧ���������������һ�������ȥ��KI��Ӧ��������������ɵ��뱽�ӷ�Ӧ����������������һ���������뱽�ӷ�Ӧ�꣬������ʣ�������KI��Ӧ�����з�Ӧ����ʱ������Һ�Ի�ɫ˵�����ӷ�Ӧ�꣬������ʣ�࣬�Ա���KI��Ӧ����ԭ��Ϊ�������ɵ�Br2���ˮ�б�����ȫ��Ӧ������Һ��ɫΪ��ɫ��˵����Br2ʣ�࣬ʣ��Br2�����KI��Ӧ���Ӷ���Ӽ��㱽�����ĵ�Br2��
��5�����з�ӦΪKBrO3 + 5KBr + 3H2SO4=3K2SO4+ 3Br2 + 3H2O��֪3n(KBrO3)=n1(Br2)������Br2�����뱽�ӷ�Ӧ��ʣ���������Ϊn2(Br2)��n1(Br2)>n2(Br2)���ڢ��з�ӦΪBr2+2KI=I2+2KBr����ʣ������ȫ��Ӧ����n(KI)�� 2n2(Br2)����֪n(KI)��6n(KBrO3)�������n(KI)��6n(KBrO3)��KIһ��������
��6�����к������Һ�ڼ�����ۣ���Һ����ɫ������Na2S2O3��Һ���룬��ɫ��dzֱ����ʧ��������������һ��Na2S2O3����Һʱ����Һ����ɫ��Ϊ��ɫ����30s����ɫ��
��7��n(BrO3-)=av1��10-3mol�����ݷ�ӦBrO3- + 5Br- + 6H+ = 3Br2 + 3H2O��֪n(Br2)=3av1��10-3mol����ֱ��뱽�Ӻ�KI��Ӧ���ȼ�����KI���ĵ����������Ϊn1(Br2)������I2+2Na2S2O3=2NaI+Na2S4O6��֪I2~2Na2S2O3����Br2+2I-=I2+2Br-��֪Br2~ I2���ɵ�Br2~2Na2S2O3��n(Na2S2O3)= bv3��10-3mol��n1(Br2)=![]() bv3��10-3mol���ټ����ɱ������ĵ����������Ϊn2(Br2)= n(Br2)- n1(Br2)=( 3av1-
bv3��10-3mol���ټ����ɱ������ĵ����������Ϊn2(Br2)= n(Br2)- n1(Br2)=( 3av1-![]() bv3) ��10-3mol����������ˮ��Ӧ�ļ�������ϵΪ3Br2~C6H5OH��n(C6H5OH)=
bv3) ��10-3mol����������ˮ��Ӧ�ļ�������ϵΪ3Br2~C6H5OH��n(C6H5OH)=![]() n2(Br2)=(av1-
n2(Br2)=(av1-![]() bv3)��10-3mol����ˮ�б��ӵĺ���=
bv3)��10-3mol����ˮ�б��ӵĺ���=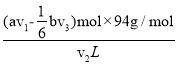 =
=![]() mol��
mol��
��8���������ɵ����뱻���Ӻ�KI��ȫ��Ӧ���������лӷ��ԣ���Ӧʱ�����ܱ������н��С�

����Ŀ��ij�о���ѧϰС��ͬѧ����NaHCO3��KHCO3��ɵ�ij���Ȼ�������ʵ��,�����������(��������ʵ���Ũ�����):
50mL���� | 50mL���� | 50mL���� | |
m(�����) | 9.2g | 15.7g | 27.6g |
��״����,V(CO2) | 2.24L | 3.36L | 3.36L |
(1)��������ʵ���Ũ��Ϊ_________��
(2)�������,n(NaHCO3):n(KHCO3)=_________��
����Ŀ���������ӷ���ʽ������������Ӧʵ��������ǣ� ��
ʵ������ | ���ӷ���ʽ | |
A | ��������þ����Һ�еμ��Ȼ����Һ�������ܽ� |
|
B | ���ˮ�еμӱ����Ȼ�����Һ�õ����ɫҺ�� |
|
C | ��������ʹ���Ը��������Һ��ɫ |
|
D | ������������ϡ���� |
|
A. AB. BC. CD. D