��Ŀ����
����Ŀ��������ƽ�������ƾ������֢����ͨ�����·����ϳɲ��ַ�Ӧ������ȥ)
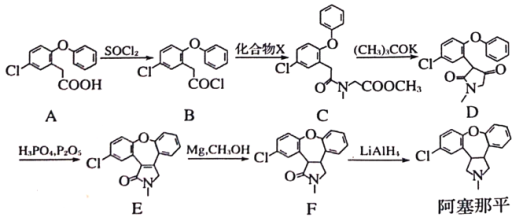
(1)������ƽ�еĺ�������������Ϊ___________����F��������ƽ�ķ�Ӧ����Ϊ___________������A�ķ���ʽΪ___________��
(2)A����B�Ĺ�������SO2������д��A��B��Ӧ����ʽ______________________��
(3)������X�ķ���ʽΪC4H9NO2����X�Ľṹ��ʽΪ______________________��
(4)��D����E�Ĺ������ȷ����ӳɷ�Ӧ��������ȥ��Ӧ���ӳɷ�Ӧ�����ɵ��м���Ľṹ��ʽΪ___________��
(5)д��ͬʱ��������������B��һ��ͬ���칹��Ľṹ��ʽ��__________________��
I���ڷ��㻯��������к���5�ֲ�ͬ��ѧ�������⣻
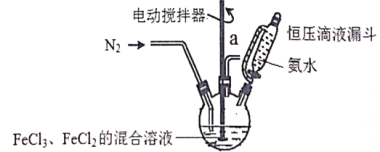
���ܷ���������Ӧ��ˮ�ⷴӦ��ˮ���������FeCl3��Һ������ɫ��Ӧ��
(6)���������ϳ����̣�д����CH3NH2��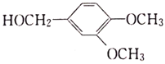 Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�����(���Լ�����)______________________��
�ĺϳ�����(���Լ�����)______________________��
���𰸡��Ѽ� ��ԭ��Ӧ C14H11O3Cl 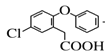 +SOCl2 ��
+SOCl2 �� +SO2+HCl CH3OOCCH2NHCH3
+SO2+HCl CH3OOCCH2NHCH3 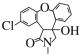
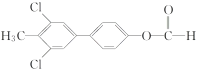 (��
(��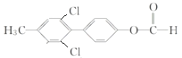
 )
) ![]()
![]()
![]()
![]()
![]()
��������
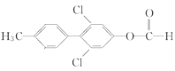
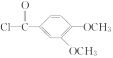
(1)���ݰ�����ƽ�Ľṹ��ʽ��֪���к�������������Ϊ�Ѽ�����F��LiAlH4������ԭ��Ӧ��Ϊ������ƽ������A�ṹ��ʽ��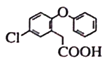 ������ʽΪC14H11O3Cl��
������ʽΪC14H11O3Cl��
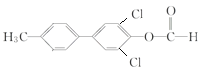
(2)A�� ����SOCl2��Ӧ����SO2��ͬʱ����B��
����SOCl2��Ӧ����SO2��ͬʱ����B��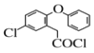 ����A��B��Ӧ����ʽΪ��
����A��B��Ӧ����ʽΪ�� +SOCl2=SO2+
+SOCl2=SO2+ ��
��
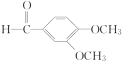
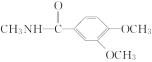
(3)������X�ķ���ʽΪC4H9NO2�����ݻ�����B��C�Ľṹ����������غ㶨�ɣ���֪X�Ľṹ��ʽΪCH3OOCCH2NHCH3��
(4)��D�ṹ��ʽ��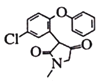 ���������Ԫ������H2�����ӳɷ�Ӧ����
���������Ԫ������H2�����ӳɷ�Ӧ���� ���������ٷ�����ȥ��Ӧ����ȥһ��H2O���õ�E
���������ٷ�����ȥ��Ӧ����ȥһ��H2O���õ�E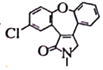 ��
��
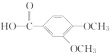
(5)B�ṹ��ʽ��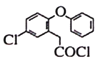 ������ͬ���칹���У�����������I���ڷ��㻯��������к���5�ֲ�ͬ��ѧ�������⣬˵�����б�������������5��Hԭ�ӣ����ܷ���������Ӧ��ˮ�ⷴӦ��ˮ���������FeCl3��Һ������ɫ��Ӧ��˵������ȩ���ͷ��ǻ��γɵ�������������ʵĽṹ��ʽ��
������ͬ���칹���У�����������I���ڷ��㻯��������к���5�ֲ�ͬ��ѧ�������⣬˵�����б�������������5��Hԭ�ӣ����ܷ���������Ӧ��ˮ�ⷴӦ��ˮ���������FeCl3��Һ������ɫ��Ӧ��˵������ȩ���ͷ��ǻ��γɵ�������������ʵĽṹ��ʽ�� ����
���� ��
�� ��
�� ��
��
(6)��CH3NH2��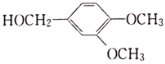 Ϊԭ���Ʊ�
Ϊԭ���Ʊ�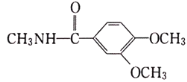 �ĺϳ�����Ϊ��
�ĺϳ�����Ϊ��![]()
![]()

![]()

![]()

![]()
 ��
��

����Ŀ������������������Ҫ��ʩ�л�������ʱ��ͨ���ơ�������ҵͣҵ�����ﳾ��Ⱦ���Ƶȡ�
��1��PM2.5�ǻ������ż�������������Ҫָ�ꡣ��ijPM2.5����������ˮ�����Ƴɴ������������������������(OH�����Բ���)�������ƽ��Ũ�����±���
�������� | Na�� | NH4+ | SO42- | NO3- |
Ũ��(mol/L) | 2.0��10��6 | 2.8��10��5 | 3.5��10��5 | 6.0��10��5 |
��������pHΪ________________________��
��2��һ�������£���CO��H2�ϳ������ԴCH3OH�����Ȼ�ѧ����ʽΪCO(g)��2H2(g) CH3OH(g)����H��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��

�ٸÿ��淴Ӧ�Ħ�H__________0(�>����<������)��A��B��C�����Ӧ��ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵ��______________��ѹǿ��p1__________p2(�>����<������)����T1�����£���D�㵽B������У������淴Ӧ����֮��Ĺ�ϵ��v(��)___________v(��)(�>����<������)��
�����ں��º��������½���������Ӧ���ܱ�ʾ�ÿ��淴Ӧ�ﵽƽ��״̬����_________(����ĸ)��
A.CO������������ֲ���
B�������ڻ��������ܶȱ��ֲ���
C�������ڻ�������ƽ��Ħ���������ֲ���
D����λʱ��������CO��Ũ�ȵ�������CH3OH��Ũ��
�����ѹ�ܱ������г���2 mol CO��4 mol H2����p2��T2�����´ﵽƽ��״̬C�㣬��ʱ�����ݻ�Ϊ2 L�����ڸ������·�Ӧ��ƽ�ⳣ��KΪ_________________________��