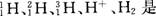��Ŀ����
��9�֣�������Ԫ��A��B��C��D��E��ԭ����������������Ԫ��������Ϣ���±���
��Dԭ�ӽṹʾ��ͼΪ____________��A�����ڱ��е�λ��Ϊ________________��
���õ���ʽ��ʾB��C�γɵĻ����� ___________��
��B��D�����ӵİ뾶��СΪ__________��(�����ӷ��ź͡�>������=����<����ʾ)
��Ԫ�طǽ�����ǿ���Ƚ��кܶ��������B��E�ķǽ�����ǿ�����о������в����е���_________������ţ���
a.�Ƚ����ֵ��ʵ���ɫ b.�Ƚ��⻯����ȶ��� c.������Ԫ�������ڱ���λ��
d.�Ƚ�ԭ�Ӱ뾶��С e.�Ƚ�����������Ӧˮ���������
��EԪ��������������Ԫ���е�һ��Ԫ���γɹ��ۻ���������е�ԭ�Ӹ�����Ϊ1:3����Է�������Ϊ120.5��������ʵĽṹʽΪ___________________��
| Ԫ�ر�� | Ԫ��������Ϣ |
| A | ������ۺ�����۵ľ���ֵ֮��Ϊ2 |
| B | ԭ�Ӻ���P�Dz��������S�Dz��������1 |
| C | 1molC���������� ˮ��Ӧ���ڱ�״��������11.2LH2 ˮ��Ӧ���ڱ�״��������11.2LH2 |
| D | ԭ�������������������������� |
| E | ��һ�������ӵĵ��Ӳ�ṹ��Arԭ����ͬ |
��Dԭ�ӽṹʾ��ͼΪ____________��A�����ڱ��е�λ��Ϊ________________��
���õ���ʽ��ʾB��C�γɵĻ����� ___________��
��B��D�����ӵİ뾶��СΪ__________��(�����ӷ��ź͡�>������=����<����ʾ)
��Ԫ�طǽ�����ǿ���Ƚ��кܶ��������B��E�ķǽ�����ǿ�����о������в����е���_________������ţ���
a.�Ƚ����ֵ��ʵ���ɫ b.�Ƚ��⻯����ȶ��� c.������Ԫ�������ڱ���λ��
d.�Ƚ�ԭ�Ӱ뾶��С e.�Ƚ�����������Ӧˮ���������
��EԪ��������������Ԫ���е�һ��Ԫ���γɹ��ۻ���������е�ԭ�Ӹ�����Ϊ1:3����Է�������Ϊ120.5��������ʵĽṹʽΪ___________________��
��
 ���ڶ����ڡ��ڢ�A�壻 ��
���ڶ����ڡ��ڢ�A�壻 �� ��(��1��)
��(��1��)
��r��F-��>r��Al3+���� ��ae�� �� ��(��2��)
��(��2��)

 ���ڶ����ڡ��ڢ�A�壻 ��
���ڶ����ڡ��ڢ�A�壻 �� ��(��1��)
��(��1��)��r��F-��>r��Al3+���� ��ae�� ��
 ��(��2��)
��(��2��)��

��ϰ��ϵ�д�
�����Ŀ
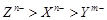
 5��
5�� ����ͬʱ����ˮ�У�����������ɫ���壬�йصĻ�ѧ����ʽΪ���� ��������������������������������������������
����ͬʱ����ˮ�У�����������ɫ���壬�йصĻ�ѧ����ʽΪ���� ��������������������������������������������