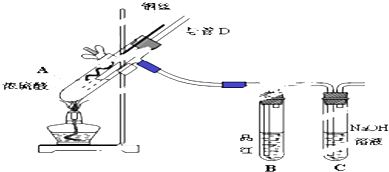
��Ŀ����
19����ȸʯ��ʯ������Ȼ����ڵ�����̼������ͭ�����ǵĻ�ѧ��ɿɱ�ʾΪ��xCuCO3•yCu��OH��2��x��yΪ��������x��2��y��2������1����֪��ȸʯ�Ļ�ѧ���ΪCuCO3•Cu��OH��2��1mol��ȸʯ��������ᷴӦʱ����ȸʯ���õ�HCl�����ʵ���Ϊ4mol�����ɵ�CO2�����ʵ���Ϊ1mol��
��2��ʯ����������ᷴӦʱ��ʯ����õ�HCl�����ʵ��������ɵ�CO2�����ʵ���֮��Ϊ3��1��ͨ����������ʯ��Ļ�ѧ��ɣ�
��3�����п�ȸʯ��ʯ������Ʒ��ȡ���ݵ���������Ʒ����һ���м���������ᣬ����CO2 3.36L����״���£���������һ����Ʒʹ����ȫ�ֽ⣬�õ�CuO 20g����ͨ������ȷ���û�����п�ȸʯ��ʯ������ʵ���֮�ȣ�
���� ��1��������Ӧ��CuCO3•Cu��OH��2+4HCl�T2CuCl2+CO2��+3H2O�����ݷ�Ӧ�Ļ�ѧ����ʽ���м��㣻
��2��������Ӧ��xCuCO3•yCu��OH��2+��2x+2y��HCl�T��x+y��CuCl2+xCO2��+��x+2y��H2O���������ѧ��ɿɱ�ʾΪxCuCO3•yCu��OH��2��x��y��ϵ���ٸ���x��yΪ��������x��2��y��2�������������
��3�����ȸʯ��ʯ������ʵ����ֱ�Ϊamol��bmol�����ݷ���ʽ��ʾ�����ɵĶ�����̼��CuO���з��̼�����
��� �⣺��1��CuCO3•Cu��OH��2+4HCl�T2CuCl2+CO2��+3H2O
1 4 1
1mol n��HCl�� n��CO2��
���ԣ�n��HCl��=$\frac{1mol��4}{1}$=4mol��
n��CO2��=$\frac{1mol��1}{1}$=1mol��
�ʴ�Ϊ��4��1��
��2��������Ӧ��xCuCO3•yCu��OH��2+��2x+2y��HCl�T��x+y��CuCl2+xCO2��+��x+2y��H2O��ʯ����õ����������ɵ�CO2�����ʵ�����Ϊ3��1����2x+2y����x=3��1�����x=2y��
����x��y��������x��2��y��2����x=2��y=1��
��ʯ��Ļ�ѧʽ�ɱ�ʾΪ��2CuCO3•Cu��OH��2��
��ʯ��Ļ�ѧ���Ϊ2CuCO3•Cu��OH��2��
��2�����ȸʯ��ʯ������ʵ����ֱ�Ϊamol��bmol����
CuCO3•Cu��OH��2+4HCl�T2CuCl2+CO2��+3H2O
1 1
amol amol
2CuCO3•Cu��OH��2+6HCl�T3CuCl2+2CO2��+4H2O
1 2
bmol 2bmol
CuCO3•Cu��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO+CO2��+H2O
1mol 160g
amol 160ag
2CuCO3•Cu��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$3CuO+2CO2��+H2O
1mol 240
bmol 240bg
��a+2b=$\frac{3.36}{22.4}$��160a+240b=20��������ã�a=0.05��b=0.05��
�û�����п�ȸʯ��ʯ������ʵ���֮��=0.05mol��0.05mol=1��1��
�𣺸û�����п�ȸʯ��ʯ������ʵ���֮��Ϊ1��1��
���� ���⿼�鸴�ӻ�ѧʽ���ƶϡ���ѧ��Ӧ����ʽ�ļ��㣬��Ŀ�Ѷ��еȣ�ע����ݷ���ʽ���м���ķ�������ȷ���ӻ�ѧʽ��ȷ������������������ϴ��������ѧ���ķ�����������ѧ����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��ͭ����Ʒ�Ʋ����������Ʒ������ǰ���������� | |
| B�� | CaCO3��s���TCaO��s��+CO2��g�������²����Է����У�˵���÷�Ӧ�ġ�H��0 | |
| C�� | N2��g��+3H2��g���T2NH3��g����H��0�������¶ȣ���Ӧ����v��H2 ����H2��ƽ��ת���ʾ����� | |
| D�� | ˮ�����ӻ�����Kw�����¶ȵ����߶�����˵��ˮ�ĵ����Ƿ��ȷ�Ӧ |
| A�� | n+11 | B�� | n-5 | C�� | n+3 | D�� | n+5 |
| A�� | ú�ĸ����ʯ�͵ķ�������ѧ�仯 | |
| B�� | ���ᱵ��ҽѧ���������ͣ������Ӷ������� | |
| C�� | ̼-14��������������ļ�����̼-14��̼-12��Ϊͬ�������� | |
| D�� | ������ע��Һ���ܲ��������ЧӦ�������ڽ��� |
| A�� | 10 g H2��10 g O2 | B�� | 5.6 L N2����״������22 g CO2 | ||
| C�� | 9 g H2O��0.5 mol Br2 | D�� | 224 mL H2��0.01 mol N2 |
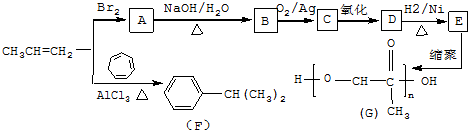
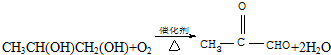 ��C����������Ӧ�Ļ�ѧ����ʽ
��C����������Ӧ�Ļ�ѧ����ʽ ��
��