��Ŀ����
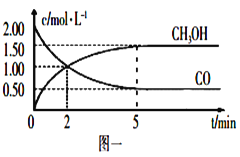
����Ŀ��������ⷨ���Ѱ۲����ķ�Һ��������(Fe2O3)�Ͳ�Ѫ������������������ҵ��������ͼ��ʾ:
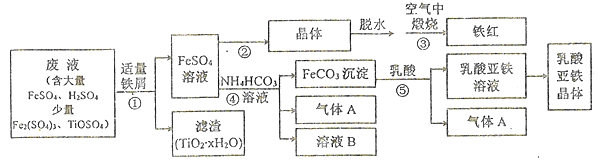
��֪;TiOSO4�ڴ���ˮ�м��������¿���ȫˮ���ΪTiO2��xH2O������
�밴Ҫ��ش���������:
��1������ټ���������м��Ŀ����_________��TiOSO4��ȫˮ��Ļ�ѧ����ʽΪ_________��
��2������ڵ�ϵ�в����У���Ҫ����__________��_________�����£��ٽ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ϴ��Һѡ��ϡ�������ˮ���ŵ���__________��
��3��д���������Ļ�ѧ����ʽ: _________��
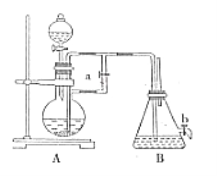
��4��ij��ȤС��ѧ������м��ϡ���ᡢNH4HCO3��ҺΪԭ�ϣ�������ͼװ�ã����ò���ܷ�Ӧ���ﵽ�Ʊ�FeCO3��Ŀ�ġ�
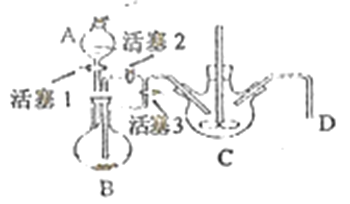
i.NH4HCO3��ҺӦʢ����װ��(����ĸ) _______�С���װ�����漰����Ҫ��Ӧ�����ӷ���ʽ_________��
ii.��ɲ���ܷ�Ӧ�IJ�����__________��
��5���ⶨ����������þ�����FeSO4��7H2O����������:
��ȡag������Ʒ�����100.00mL��Һ��ȡ��25.00mL��Һ���������ữ��0.1000mol/LKMnO4����Һ�ζ�(���ʲ���KMnO4��Һ��Ӧ)��ƽ����Чʵ��ζ�����KMnO4��Һ�����ƽ��ֵΪ20.00mL�������þ�����FeSO4��7H2O����������Ϊ����M(FeSO4��7H2O)=bg/mol���ú�a��b�ı���ʽ��ʾ��_________��
���𰸡� ��Fe3+��ԭΪFe2+,���IJ���H+ TiOSO4+(x+1)H2O(����) ![]() TiO2��xH2O��+H2SO4 ������Χ�����۹��� ������� ���پ�����ܽ⣬��߲��� 4FeSO4+O2
TiO2��xH2O��+H2SO4 ������Χ�����۹��� ������� ���پ�����ܽ⣬��߲��� 4FeSO4+O2![]() 2Fe2O3+4SO3 C Fe2++2HCO3-=FeCO3��+H2O+CO2�� ������D���ܵ��������崿���رջ���3,����2 (0.04b/a)x100%
2Fe2O3+4SO3 C Fe2++2HCO3-=FeCO3��+H2O+CO2�� ������D���ܵ��������崿���رջ���3,����2 (0.04b/a)x100%
����������1����ʵ��Ŀ��֪Ҫ�����������͵ð�Fe3+��ԭΪFe2������ټ���������м��Ŀ���ǽ�Fe3+��ԭΪFe2+,ͬʱ�������Ժ��ᷴӦ���IJ���H+��TiOSO4��ȫˮ������TiO2��xH2O��H2SO4���仯ѧ����ʽΪTiOSO4+(x+1)H2O(����) ![]() TiO2��xH2O��+H2SO4��
TiO2��xH2O��+H2SO4��
��2��Ϊ�˷�ֹ�ڼ��ȹ�����Fe2+��������ˮ�⣬���Բ���ڲ�������Ҫ�����ڵ�����Χ�����۹�������������������½�������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ϴ��Һѡ��ϡ�������ˮ���ŵ��м��پ�����ܽ⣬��߲��ʡ�
��3�������������ɵĵ�FeSO4����������������ķ�Ӧ���䷴Ӧ�Ļ�ѧ����ʽ: 4FeSO4+O2![]() 2Fe2O3+4SO3��
2Fe2O3+4SO3��
i����ܷ�Ӧ��FeSO4��NH4HCO3��Ӧ��ȡFeCO3������NH4HCO3��ҺӦʢ����Cװ���С���װ����Ҫ��Ӧ�����ӷ���ʽFe2++2HCO3-=FeCO3��+H2O+CO2����
ii.��Ϊ�������Ӻܲ��ȶ������ױ��������������Ҫ��ɲ���ܱ������ų�װ���еĿ��������Դ�ɲ���ܷ�Ӧ�IJ����Ǵ�����D���ܵ��������崿���رջ���3,����2������
��5���������ӻᱻ�����������Ϊ����������, KMnO4����ԭΪ+2�۵�������,���ݵ����غ�,��FeSO4��7H2O- KMnO4,����0.1000mol/LKMnO4��Һ20.00mL,���Ծ�����FeSO4��7H2O����������=[(0.1000mol/L![]() 0.02
0.02![]() b
b![]() )/ag]
)/ag]![]() 100%= (0.04b/a)x100%����˱�����ȷ����: (0.04b/a)x100%��
100%= (0.04b/a)x100%����˱�����ȷ����: (0.04b/a)x100%��