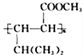ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ»ή“Κ÷–ΒΡΜ·―ßΖ¥”Π¥σΕύ «άκΉ”Ζ¥”ΠΓΘΗυΨί“Σ«σΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©―ΈΦνΒΊ(Κ§ΫœΕύNa2CO3ΓΔNaCl)≤Μάϊ”Ύ÷≤Έο…ζ≥ΛΘ§ ‘”ΟάκΉ”ΖΫ≥Χ Ϋ±μ Ψ―ΈΦνΒΊ≥ Φν–‘ΒΡ‘≠“ρΘΚ____________________________________ΓΘ
Θ®2Θ©“―÷ΣΥ°¥φ‘ΎΤΫΚβ2H2O![]() H3OΘΪΘΪOHΘ≠Θ§œρΥ°÷–Φ”»κNaHSO4ΙΧΧεΘ§Υ°ΒΡΒγάκΤΫΚβ________“ΤΕ·Θ§ΥυΒΟ»ή“Κœ‘________–‘ΓΘ
H3OΘΪΘΪOHΘ≠Θ§œρΥ°÷–Φ”»κNaHSO4ΙΧΧεΘ§Υ°ΒΡΒγάκΤΫΚβ________“ΤΕ·Θ§ΥυΒΟ»ή“Κœ‘________–‘ΓΘ
Θ®3Θ©»τ»ΓpHΓΔΧεΜΐΨυœύΒ»ΒΡNaOH»ή“ΚΚΆΑ±Υ°Ζ÷±πΦ”Υ°œΓ Άm±ΕΓΔn±ΕΘ§œΓ ΆΚσpH»‘œύΒ»Θ§‘ρm________ n(ΧνΓΑΘΨΓ±ΓΑΘΦΓ±ΜρΓΑΘΫΓ±)ΓΘ
Θ®4Θ©≥ΘΈ¬œ¬Θ§‘ΎpHΘΫ6ΒΡCH3COOH”κCH3COONaΒΡΜλΚœ»ή“Κ÷–Θ§”…Υ°Βγάκ≥ωά¥ΒΡc(OHΘ≠)ΘΫ________molΓΛLΘ≠1ΓΘ
Θ®5Θ©“―÷ΣΘΚ≥ΘΈ¬œ¬Θ§NH3ΓΛH2OΒΡΒγάκΤΫΚβ≥Θ ΐKbΘΫ1.75ΓΝ10Θ≠5Θ§H2CO3ΒΡΒγάκΤΫΚβ≥Θ ΐKa1ΚΆKa2Ζ÷±πΈΣ4.4ΓΝ10Θ≠7ΚΆ5.6ΓΝ10Θ≠11, “άΨί…œ ωKbΘΨ__________(ΧνΓΑKa1Γ± ΜρΓΑ Ka2Γ±)Ω…ΆΤ÷ΣNH4HCO3»ή“ΚΒΡΥαΦν–‘ «________–‘ΓΘ
ΓΨ¥πΑΗΓΩ CO![]() ΘΪH2O
ΘΪH2O![]() HCO
HCO![]() ΘΪOHΘ≠ œρΉσ Υα ΘΦ 1ΓΝ10Θ≠8 Ka1 Φν
ΘΪOHΘ≠ œρΉσ Υα ΘΦ 1ΓΝ10Θ≠8 Ka1 Φν
ΓΨΫβΈωΓΩΘ®1Θ©ΧΦΥαΡΤΥ°ΫβΘ§»ή“Κœ‘Φν–‘Θ§“ρ¥Υ―ΈΦνΒΊ≥ Φν–‘ΒΡ‘≠“ρ «CO32Θ≠ΘΪH2O![]() HCO3Θ≠ΘΪOHΘ≠ΓΘΘ®2Θ©œρΥ°÷–Φ”»κNaHSO4ΙΧΧεΘ§«βάκΉ”≈®Ε»‘ω¥σΘ§Υ°ΒΡΒγάκΤΫΚβœρΉσ“ΤΕ·Θ§ΥυΒΟ»ή“Κœ‘Υα–‘ΓΘΘ®3Θ©œΓ ΆΙΐ≥Χ÷–¥ΌΫχ“ΜΥ°ΚœΑ±ΒΡΒγάκΘ§«β―θΗυΒΡΈο÷ ΒΡΝΩ‘ωΦ”Θ§Υυ“‘“Σ ΙœΓ ΆΒΡΚσΒΡpHœύΒ»Θ§‘ρΑ±Υ°œΓ ΆΒΡ±Ε ΐ¥σΘ§Φ¥mΘΦnΓΘΘ®4Θ©≥ΘΈ¬œ¬Θ§‘ΎpHΘΫ6ΒΡCH3COOH”κCH3COONaΒΡΜλΚœ»ή“Κ÷–¥ΉΥαΒΡΒγάκ≥ΧΕ»¥σ”Ύ¥ΉΥαΗυΒΡΥ°Ϋβ≥ΧΕ»Θ§“ρ¥Υ”…Υ°Βγάκ≥ωά¥ΒΡc(OHΘ≠)ΘΫ
HCO3Θ≠ΘΪOHΘ≠ΓΘΘ®2Θ©œρΥ°÷–Φ”»κNaHSO4ΙΧΧεΘ§«βάκΉ”≈®Ε»‘ω¥σΘ§Υ°ΒΡΒγάκΤΫΚβœρΉσ“ΤΕ·Θ§ΥυΒΟ»ή“Κœ‘Υα–‘ΓΘΘ®3Θ©œΓ ΆΙΐ≥Χ÷–¥ΌΫχ“ΜΥ°ΚœΑ±ΒΡΒγάκΘ§«β―θΗυΒΡΈο÷ ΒΡΝΩ‘ωΦ”Θ§Υυ“‘“Σ ΙœΓ ΆΒΡΚσΒΡpHœύΒ»Θ§‘ρΑ±Υ°œΓ ΆΒΡ±Ε ΐ¥σΘ§Φ¥mΘΦnΓΘΘ®4Θ©≥ΘΈ¬œ¬Θ§‘ΎpHΘΫ6ΒΡCH3COOH”κCH3COONaΒΡΜλΚœ»ή“Κ÷–¥ΉΥαΒΡΒγάκ≥ΧΕ»¥σ”Ύ¥ΉΥαΗυΒΡΥ°Ϋβ≥ΧΕ»Θ§“ρ¥Υ”…Υ°Βγάκ≥ωά¥ΒΡc(OHΘ≠)ΘΫ![]() ΓΘΘ®5Θ©”…”ΎKbΘΨKa1Θ§Υυ“‘ΧΦΥα«βΗυΒΡΥ°Ϋβ≥ΧΕ»¥σ”ΎοßΗυΒΡΥ°Ϋβ≥ΧΕ»Θ§‘ρNH4HCO3»ή“Κœ‘Φν–‘ΓΘ
ΓΘΘ®5Θ©”…”ΎKbΘΨKa1Θ§Υυ“‘ΧΦΥα«βΗυΒΡΥ°Ϋβ≥ΧΕ»¥σ”ΎοßΗυΒΡΥ°Ϋβ≥ΧΕ»Θ§‘ρNH4HCO3»ή“Κœ‘Φν–‘ΓΘ

 ≈ύ”≈»ΐΚΟ…ζœΒΝ–¥πΑΗ
≈ύ”≈»ΐΚΟ…ζœΒΝ–¥πΑΗΓΨΧβΡΩΓΩœ¬ΆΦΥυ ΨΒΡ Β―ιΘ§≤ΜΡή¥οΒΫ Β―ιΡΩΒΡΒΡ «Θ®Ης―Γœν÷–Ε‘±»»ή“ΚΒΡ≈®Ε»ΧεΜΐΨυœύΆ§Θ©( )
Β―ιΖΫΑΗ |
|
|
|
|
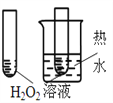
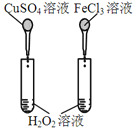
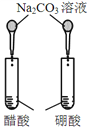
ΡΩΒΡ | AΘ°―ι÷Λ…ΐΗΏΈ¬Ε»Ω…Φ”ΩλH2O2Ζ÷Ϋβ | BΘ°―ι÷Λ‘ω¥σΖ¥”ΠΈο≈®Ε»Ε‘ΤΫΚβΒΡ”Αœλ | CΘ°±»ΫœCu2+ΓΔFe3+Ε‘H2O2Ζ÷ΫβΥΌ¬ ΒΡ”Αœλ | DΘ°±»Ϋœ»θΥαΒΡœύΕ‘«Ω»θ |
A. A B. B C. C D. D