��Ŀ����
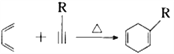
����Ŀ��E��һ�ֻ���������,��ϳ�·�����£�
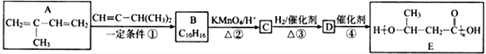
��֪����
��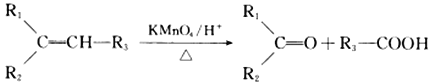 (R1��R2��R3��R����)
(R1��R2��R3��R����)
��ش��������⣺
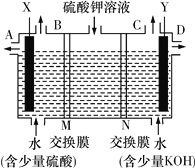
��1��A�й����ŵ�������____________������ù����ŵ��Լ�Ϊ___________________��
��2����Ӧ�ٵĻ�ѧ����ʽ�ǣ�______________________________���䷴Ӧ����Ϊ________________���ڷ�Ӧ���У������Եõ���һ�ַ���ʽΪC10H16�Ļ������ṹ��ʽΪ___________________��
��3����֪��![]() ����Ϊ��ͪ�ᣬ��C��ϵͳ����������ӦΪ____________________��
����Ϊ��ͪ�ᣬ��C��ϵͳ����������ӦΪ____________________��
��4��д����Ӧ�ܵĻ�ѧ����ʽ��______________________________��
��5��C��ͬ���칹��X��������������
�ٳ����£���̼������Һ��Ӧ�ų����壻
���ܷ���������Ӧ�������������X����________�֡���˴Ź���������������ҷ����֮��Ϊ1��1��2��2����X�Ľṹ��ʽΪ________________��
��6������E�������ϳ�·�ߣ����һ����4һ��һ3һ��ͪ��Ϊ��ʼԭ���Ʊ�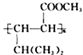 �ĺϳ�·��(���Լ���ѡ)_________________________________________��
�ĺϳ�·��(���Լ���ѡ)_________________________________________��
���𰸡� ̼̼˫�� ���CCl4��Һ  �ӳɷ�Ӧ
�ӳɷ�Ӧ 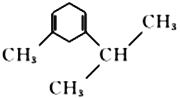 3-��ͪ��
3-��ͪ�� ![]() 2 HOOCCH2CH2CHO
2 HOOCCH2CH2CHO 
����������1�������л���A�Ľṹ��ʽ��֪��A�й�����Ϊ̼̼˫����̼̼˫���ܹ����巢���ӳɷ�Ӧ����ˮ��ɫ����˼���ù����ŵ��Լ�Ϊ���CCl4��Һ����ȷ�𰸣�̼̼˫�������CCl4��Һ��
��2��������Ϣ��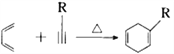 ��֪��
��֪��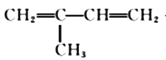 ��
��![]() �����ӳɷ�Ӧ����ѧ����ʽ�ǣ�
�����ӳɷ�Ӧ����ѧ����ʽ�ǣ�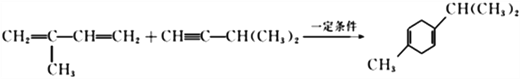 ���ڷ�Ӧ���У��������
���ڷ�Ӧ���У��������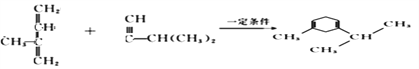 ��Ӧ�������Եõ���һ�ַ���ʽΪC10H16�Ļ������ṹ��ʽΪ
��Ӧ�������Եõ���һ�ַ���ʽΪC10H16�Ļ������ṹ��ʽΪ![]() ����ȷ�𰸣�
����ȷ�𰸣�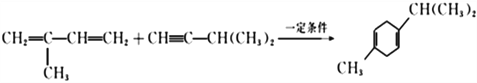 �� �ӳɷ�Ӧ ��
�� �ӳɷ�Ӧ �� 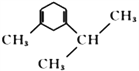 ��
��
��3��������Ϣ��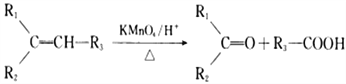 (R1��R2��R3��RΪ����)��֪���л���B
(R1��R2��R3��RΪ����)��֪���л���B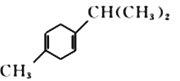 �����Ը��������Һ����ΪCH3COCH2COOH, ϵͳ����������ӦΪ3-��ͪ�����һ������Ϊ(CH3)CH-COCH2COOH����ȷ�𰸣�3-��ͪ�ᡣ
�����Ը��������Һ����ΪCH3COCH2COOH, ϵͳ����������ӦΪ3-��ͪ�����һ������Ϊ(CH3)CH-COCH2COOH����ȷ�𰸣�3-��ͪ�ᡣ
��4���л���CΪCH3COCH2COOH,�����������ӳ�����CH3CH(OH)CH2COOH, CH3CH(OH)CH2COOH��һ�������·������۷�Ӧ���ɸ߷��ӣ���ѧ����ʽ��![]() ����ȷ�𰸣�
����ȷ�𰸣�![]() ��
��
��5���л���C�ṹ��ʽΪ CH3COCH2COOH,������ͬ���칹���X���������������ٳ����£���̼������Һ��Ӧ�ų����壬�����Ȼ������ܷ���������Ӧ������ȩ������˷����������л����У�OHC-CH2CH2COOH��OHC-CH(CH3)COOH������2�֣���˴Ź���������������ҷ����֮��Ϊ1��1��2��2����X�Ľṹ��ʽΪ HOOCCH2CH2CHO����ȷ�𰸣�2��HOOCCH2CH2CHO��
��6��(CH3)2CHCOCH2COOH�����������ӳɷ�Ӧ����(CH3)2CHCH(OH)-CH2COOH ��(CH3)2CHCH(OH)-CH2COOH��Ũ���������¼��ȷ�����ȥ��Ӧ����(CH3)2CHCH=CHCOOH, (CH3)2CHCH=CHCOOH��״�������������(CH3)2CHCH=CHCOOCH3��(CH3)2CHCH=CHCOOCH3��һ�������·����Ӿ����ɸ߷��ӣ��ϳ��������£�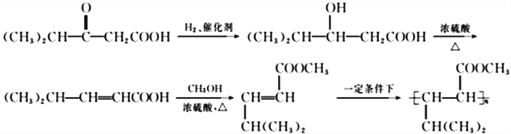 ����ȷ�𰸣�
����ȷ�𰸣� ��
��

 �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�����Ŀ����ҵ�ϲ���FeSO4��NaOHΪ��Ҫԭ�ϡ��ڼ���������ͨ��������������Ʊ����ŷ�Fe3O4����ԭ������:
��FeSO4 +2NaOH==Fe(OH)2�� +Na2SO4
��4Fe(OH)2+O2 +2H2O= =4Fe(OH)3��
��Fe(OH)2+2Fe(OH)3![]() Fe3O4+4H2O
Fe3O4+4H2O
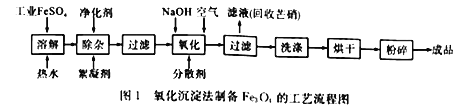
���������ڲ�ͬ�¶��µ��ܽ��:
�¶�/�� | 0 | 10 | 30 | 50 | 60 | 70 | 80 | 90 |
�ܽ��/g | 14.0 | 17.0 | 25.0 | 33.0 | 35.3 | 33.0 | 30.5 | 27.0 |
I.���������ۣ�
(1)�ܽ�ʱ��������ˮ���¶�ԼΪ___________��Ŀ����_______________________________________��
(2)����Ʒâ���Ļ�ѧʽ��_______________________��
(3)Ϊ�˼����Ʒ�Ƿ�ϴ�Ӹɾ�����Ҫ���е�ʵ�ղ�����________________________________��
II.�����ȷ����
������ʾ:��������������������ʱ�ֽ���������������(���ϼ۲���);
��Fe2O3��CO��Ӧ�����¶����߶����еģ�������Fe3O4 ,������FeO(��ɫ)���������Fe��
Ϊȷ�������Ϸ����ƵõIJ�Ʒ�Ĵ��ȣ���ȡ23.28g����Ʒ����ͼ2װ�ý���ʵ��̽����
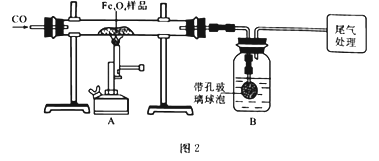
(1)����ͬѧ��ͨ������Bװ�÷�Ӧǰ��������仯�����㲢ȷ�ϸ���Ʒ�е����ʡ�B�е�����Լ���________(����ĸ)��B�д��ײ������ݵ�������____________________________________��
A.����ʯ��ˮ B.��������Ũ��Һ C.ϡ���� D.ˮ
(2)����ʵ������У�CO�������Ϊ��Ӧ���⣬�������û���:
��ʵ�鿪ʼʱ���ž�װ���еĿ�������ֹ����ʱ������ը��
��ֹͣ���Ⱥ���ͨCO���壬��ֹ�����ﱻ��������ֹB�е���Һ������A�У�������_______�������ʵ��ľ�ȷ�ȡ�
(3)���������ⶨ�����Ƴ���Ӧ������Aװ���в������ڵĹ����������¶ȵı仯���ߣ���ͼ3:
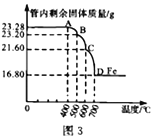
����Ʒ�к��е����ʳɷ���_____________________(�ѧʽ)��
����Ʒ�����ʵ���������Ϊ_____ %(����2λС��)��