��Ŀ����
����Ŀ����������һ����ɫ���壬������ˮ����������������������ƾ���ӡˢ��ҽҩ��Ⱦë���ȣ�Ҳ���ڵ��ӹ�ҵ�����������ȶ����������·�Ӧ��
��2AgNO3(s)��2Ag(s)+ 2NO2(g)+O2(g) ��H1��0
��2NO2(g) ![]() N2O4(g) ��H2��0
N2O4(g) ��H2��0
��1��ʵ����������������Һ�ķ����ǣ���һ������������������Ũ�����У���ˮϡ����ָ�����������������������___________________��
��2��2AgNO3(s) ��2Ag(s)+N2O4(g)+O2(g) ��H��______________ (�ú���H1����H2��ʽ�ӱ�ʾ)��
��3���¶�T1ʱ����0.5L�ĺ����ܱ�������Ͷ��3.4 g AgNO3(s)����ȫ�ֽ��û������������ʵ�����n)��ʱ��(t)�Ĺ�ϵ��ͼ��ʾ��
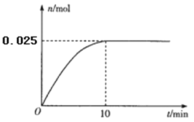
�����������˵����ϵ�ﵽƽ��״̬����_________(����ĸ)
a.�����������ٸı� b.O2��Ũ�Ȳ��ٸı�
c.NO2������������ٸı� d.���������ܶȲ��ٸı�
�����ﵽƽ��ʱ������������ѹǿp��0. 3MPa����Ӧ��ʼ��
10min��N2O4��ƽ����Ӧ����Ϊ___________ MPa��min��1���ڸ��¶���2NO2(g)![]() N2O4(g)��ƽ�ⳣ��Kp��___________(MPa)��1(�������2λС��)��
N2O4(g)��ƽ�ⳣ��Kp��___________(MPa)��1(�������2λС��)��
[��ʾ����ƽ��ʱ����ַ�ѹ���Ũ�ȼ����ƽ�ⳣ����ѹǿƽ�ⳣ��(Kp)����ֵķ�ѹ(P1) ��ƽ��ʱ��ѹ(p)������ֵ��������(![]() )]
)]
��ʵ���ã���������(NO2)������k��c2(NO2)��������2��(N2O4) ������k��c(N2O4)��k����k��Ϊ���ʳ���ֻ���¶�Ӱ�졣��ѧƽ�ⳣ��K�����ʳ���k����k������ѧ��ϵ��K��___________�������������¶ȸı�ΪT2ʱ��k����k������T1______T2(����������������������")
��4��NO��O2��Ӧ����NO2�ķ�Ӧ����Ϊ����һ��NO+NO![]() N2O2 ������ƽ�⣩
N2O2 ������ƽ�⣩
�ڶ���N2O2+O2��2NO2 ������Ӧ��������������ȷ����___________(����)��
A. ��(��һ��������Ӧ) ����(�ڶ����ķ�Ӧ) B.�ܷ�Ӧ�����ɵڶ�������
C. �ڶ����Ļ�ܱȵ�һ���ĸ� D.�ڶ�����N2O2��O2����ײ100%��Ч
���𰸡�����Ag+ˮ�� ��H1+��H2 c 0.006 4.17 ![]() �� BC
�� BC
��������
��1����������ˮ�⣬�ʼ���������ˮ�⣻
��2�����ݸ�˹���ɿ��Եõ�Ŀ�귽��ʽ���ʱ䣻
��3���ٸ���ƽ����ж����ݣ�ֱ���оݺͼ���оݣ�
3.4 g AgNO3�����ʵ���Ϊ0.02mol����ȫ�ֽ�������������ʵ���Ϊ0.01mol���������������ʵ���Ϊ0.02mol����ƽ��ʱ���������������ʵ���Ϊx mol�����Զ������������ʵ���Ϊ0.02-2x mol��ƽ��ʱ����������ʵ���Ϊ0.03 mol -x mol =0.025 mol��x=0.005���������x��ֵ�������������������������������ʵ������������������������ѹǿ���������ѹǿ�ı仯���Ӷ�������ʣ����ʵ���������������ʵ������������ƽ�ⳣ��Kp������������Ϣ����������(NO2)������k��c2(NO2)��������2��(N2O4) ������k��c(N2O4)�����ƽ�ⳣ��K�����������¶ȸı�ΪT2ʱ����k��=k�����Ƚ�K��T1ʱK����Դ�С�������жϳ��¶ȵĸߵͣ�
��4��A����һ��Ϊ�췴Ӧ����������һ��������Ӧ��>�����ڶ����ķ�Ӧ����
B���ܷ�Ӧ����������Ӧ������
C�����ͻ�ܣ��ɼӿ췴Ӧ���ʣ�
D���ڶ�����Ӧ����������Ч��ײ�Ĵ������١�
��1����������ˮ�⣬�ʼ���������Ag+ˮ�⣬
�ʴ�Ϊ������Ag+ˮ�⣻
��2����2AgNO3(s)��2Ag(s)+ 2NO2(g)+O2(g)��H1��0
��2NO2(g) ![]() N2O4(g) ��H2��0
N2O4(g) ��H2��0
���ݸ�˹���ɣ���+�ڿ��Եõ�2AgNO3(s)��2Ag(s)+N2O4(g)+O2(g)��H����H1+��H2��
�ʴ�Ϊ����H1+��H2��
��3����a�����۵��������ٸı�ֻ��˵����������ȫ�ֽ⣬����˵����Ӧ�ﵽƽ��״̬����A����
b������������䣬����������Ũ�Ȳ���ֻ��˵����������ȫ�ֽ⣬����˵����Ӧ�ﵽƽ��״̬����B����
c����������������������䣬˵����Ӧ�ﵽ��ƽ��״̬����C��ȷ��
d������������ȫ�ֽ�ʱ�������������������䣬���Ի��������ܶȲ��ٸı䲻��˵����Ӧ�ﵽƽ��״̬����D����
��Ϊc��
��3.4 g AgNO3�����ʵ���Ϊ0.02mol����ȫ�ֽ�������������ʵ���Ϊ0.01mol���������������ʵ���Ϊ0.02mol����ƽ��ʱ���������������ʵ���Ϊx mol�����Զ������������ʵ���Ϊ0.02-2x mol��ƽ��ʱ����������ʵ���Ϊ0.03 mol -x mol =0.025 mol��x=0.005�����ԣ���������ʵ���֮�ȵ���ѹǿ֮�ȣ����������������ʵ���Ϊ0.005mol��ռ����������ʵ�����![]() ������������ѹǿp��0. 3MPa��������������ѹǿҲռ
������������ѹǿp��0. 3MPa��������������ѹǿҲռ![]() ��ƽ��ʱ������������ѹǿΪ
��ƽ��ʱ������������ѹǿΪ![]() p��
p��![]() ��0. 3MPa=0.06 MPa����������������ѹǿ�仯Ϊ0.06 MPa��10min��N2O4��ƽ����Ӧ����Ϊ=
��0. 3MPa=0.06 MPa����������������ѹǿ�仯Ϊ0.06 MPa��10min��N2O4��ƽ����Ӧ����Ϊ=![]() =0.006 MPa��min��1�� ���ʵ���������������ʵ����������ɼ����֪��ƽ��ʱ���������������ʵ�������Ϊ0.2���������������ʵ�������Ϊ0.4�����Զ��������ķ�ѹΪ0.3MPa��0.4=0.12MPa�������������ķ�ѹΪ0.06MPa�����Է�Ӧ2NO2(g)
=0.006 MPa��min��1�� ���ʵ���������������ʵ����������ɼ����֪��ƽ��ʱ���������������ʵ�������Ϊ0.2���������������ʵ�������Ϊ0.4�����Զ��������ķ�ѹΪ0.3MPa��0.4=0.12MPa�������������ķ�ѹΪ0.06MPa�����Է�Ӧ2NO2(g) ![]() N2O4(g)�ڸ��¶��µ�ƽ�ⳣ��Kp=
N2O4(g)�ڸ��¶��µ�ƽ�ⳣ��Kp=![]() = 4.17��
= 4.17��
�ʴ�Ϊ��0.006��4.17��
����������(NO2)������k��c2(NO2)��������2��(N2O4) ������k��c(N2O4) ��2NO2(g) ![]() N2O4(g)��ƽ��ʱ������=������ ��k��c2(NO2)= k��c(N2O4)��ƽ�ⳣ��K=
N2O4(g)��ƽ��ʱ������=������ ��k��c2(NO2)= k��c(N2O4)��ƽ�ⳣ��K= ![]() =
= ![]() ����Ӧ2NO2(g)
����Ӧ2NO2(g) ![]() N2O4(g)��T1ʱ��ƽ�ⳣ��K=
N2O4(g)��T1ʱ��ƽ�ⳣ��K=![]() =
=![]() =25�����������¶ȸı�ΪT2ʱ����k��=k������K=1<25������ӦΪ���ȷ�Ӧ��������ƽ�ⳣ����С����T2>T1��
=25�����������¶ȸı�ΪT2ʱ����k��=k������K=1<25������ӦΪ���ȷ�Ӧ��������ƽ�ⳣ����С����T2>T1��
�ʴ�Ϊ��![]() ��<��
��<��
��4��A. ��һ��Ϊ�췴Ӧ������(��һ��������Ӧ)>��(�ڶ����ķ�Ӧ)����A����
B. �ڶ�����Ӧ���������ܷ�Ӧ�����ɵڶ�����������B��ȷ��
C. ���ͻ�ܣ��ɼӿ췴Ӧ���ʣ���ڶ����Ļ�ܱȵ�һ���ĸߣ���C��ȷ��
D. �ڶ�����Ӧ����������Ч��ײ�Ĵ������٣���֪N2O2��O2����ײ������100%��Ч����D����
�ʴ�Ϊ��BC��

����Ŀ����������ʵ��������������õ��Ľ�����ȷ���ǣ� ��
ѡ�� | ʵ����������� | ʵ����� |
A | ��0.1mol/L̼������Һ�е����̪����Һ��죬���ȣ���ɫ��� | ̼������������ |
B | �ⶨ������ҺpH���ò�����պȡ��Һ������pH��ֽ�ϣ���ֽ�Ժ�ɫ�� | ������Һ������ |
C | ��ͭƬ��пƬ�õ�������������CuSO4��Һ�У�пƬ������С��ͭƬ�������ӡ� | п����ԭ |
D | ���Ȼ�������Һ�е���KI��Һ����Һ�еĹ����ɰ�ɫ��Ϊ��ɫ�� | Ksp��AgCl��<Ksp��AgI�� |
A.AB.BC.CD.D