��Ŀ����
����Ŀ����֪A��B��C��D��E��ԭ��������������ǰ20��Ԫ�ء�Aԭ�Ӽ۵��Ӳ�p�����ֻ��1�����ӣ�B��C��DԪ�صĻ�̬ԭ�Ӿ�����ͬ���ܲ�����B��DԪ��ԭ�ӵ�p�ܼ��϶���1��δ�ɶԵ��ӣ�Dԭ�ӵ�һ����������3p�����3p����ѳ�����Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�E��ͬ���ڵ�һ��������С��Ԫ�ء��ش��������⣺
��1��B3+�Ľṹʾ��ͼΪ______��C������������Ӧˮ������E������������Ӧˮ���ﰴ���ʵ���1:1��Ӧ�����ӷ���ʽΪ__________��
��2����������Ԫ���м�����ǿ���ʵĵ���ʽΪ_________�� D�������ڵ�һ����������Ԫ����________��AD3��_________���ӣ�����ԡ��Ǽ��ԡ���
��3��B����Ϊ�����������壬������Bԭ�ӵ���λ��Ϊ_________.
��4��ʯī����ƽ���״�ṹ��ͬһ���е�ԭ�ӹ������������������Σ��������ڵ�E��������ã��γ�ij����ͭɫ�����ʣ����е�Ԫ��E�á���ʾ����ԭ�ӷֲ���ͼ��ʾ�������ʵĻ�ѧʽΪ_________________��

���𰸡�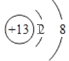 H3PO4+ OH- =H2PO4- + H2O
H3PO4+ OH- =H2PO4- + H2O![]() Ar�Ǽ���12C8K/KC8
Ar�Ǽ���12C8K/KC8
��������
A��B��C��D��E����Ԫ�����ڱ���ǰ20�ŵ�Ԫ�أ����ǵ�ԭ��������������B��C��DԪ�صĻ�̬ԭ�Ӿ�����ͬ���ܲ���������ͬһ���ڣ�B��DԪ�ص�ԭ�ӵ�p�ܼ��϶���1��δ�ɶԵ��ӣ��۲�����Ų�Ϊns2np1��ns2np5��Dԭ�ӵ�һ����������3p�����3p����ѳ�������DΪClԪ�أ�BΪAlԪ�أ�Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ��۲�����Ų�Ϊ3s23p3����CΪPԪ�أ�E��ͬ���ڵ�һ��������С��Ԫ�أ����ڵڢ�A�壬ԭ���������E���ڵ������ڣ�ΪKԪ�أ�Aԭ�ӵļ۵��Ӳ��p�����ֻ��1�����ӣ��۲�����Ų�Ϊns2np1�����ڵڢ�A�壬ԭ��������С����AΪBԪ�أ�����������֪��AΪ��Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�DΪ��Ԫ�أ�EΪ��Ԫ�ء�
��1��B3+ΪAl3+����Ľṹʾ��ͼΪ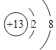 ��C������������Ӧˮ����Ϊ���ᣬE������������Ӧˮ����ΪKOH�������ʵ���1:1��Ӧ�����ӷ���ʽΪH3PO4+ OH- =H2PO4- + H2O����2������Ԫ����KԪ�صĽ�������ǿ����KOH������ǿ������ʽΪ
��C������������Ӧˮ����Ϊ���ᣬE������������Ӧˮ����ΪKOH�������ʵ���1:1��Ӧ�����ӷ���ʽΪH3PO4+ OH- =H2PO4- + H2O����2������Ԫ����KԪ�صĽ�������ǿ����KOH������ǿ������ʽΪ![]() ��Dλ�ڵ������ڣ�ͬ����������ң���һ���������ʵ�һ����������ΪAr��AD3Ϊ���Ȼ���Ϊƽ�������η��ӣ��ṹ�Գƣ����ڷǼ��Է��ӣ���3����Al�����һ��������������������������Alԭ����ͨ�������������������ϣ���ͨ��һ����������γ�8������������ÿ�����ظ�����2�Σ������ھ�����ԭ�ӵ���λ��Ϊ��3��8����2=12��
��Dλ�ڵ������ڣ�ͬ����������ң���һ���������ʵ�һ����������ΪAr��AD3Ϊ���Ȼ���Ϊƽ�������η��ӣ��ṹ�Գƣ����ڷǼ��Է��ӣ���3����Al�����һ��������������������������Alԭ����ͨ�������������������ϣ���ͨ��һ����������γ�8������������ÿ�����ظ�����2�Σ������ھ�����ԭ�ӵ���λ��Ϊ��3��8����2=12��

 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�����Ŀ����ά�ء��ȵ��غ��Լ��ض����е�Ԫ���ǣ� ��
A. C��H��O��N B. C��H��O��N��P C. C��H��O D. C��H��O��N��P��S
����Ŀ���밴Ҫ����д���пհף�
�������� | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
2 | �� | �� | �� | �� | |||
3 | �� | �� | �� | �� |
(1)��Ԫ�آ٢ڢݢޢߢ��Ӧ������������ˮ�����У�������ǿ�Ļ�����ĵ���ʽ�ǣ�_________________________________________��
(2)д��Ԫ�آڵ�����⻯��Ľṹʽ_________________________��
(3)�ܢݢޢ�����Ԫ�صļ����Ӱ뾶�Ӵ�С����____________(�����ӷ��ű�ʾ)��
(4)д��Ԫ�آ������������Ԫ�آݵ�����������ˮ���ﷴӦ�����ӷ���ʽ______��
(5)д��Ԫ�آ۵ij����⻯�����������������ˮ���ﷴӦ�Ļ�ѧ����ʽ____________��
����Ŀ��������ʵ�鼰�������Ƴ���Ӧ���۵���
ѡ�� | ʵ�� | ���� | ���� |
A | ��������ͭ�۵�Cu(NO3)2 ��Һ�е���ϡ���� | ͭ�����ܽ� | ϡ��������ͭ���ʷ�Ӧ |
B | ��H2O2��Һ�е����������Ը��������Һ | ���̲����������� | KMnO4��H2O2�ֽ�Ĵ��� |
C | ��ʢ��Ũ��ˮ����ƿ��ͨ���������������ȵIJ�˿����Һ���Ϸ� | ��˿���ֺ��ȣ���ƿ���а��̲��� | ���Ĵ�������ӦΪ���ȷ�Ӧ |
D | �������Ʒ���ȼ�ճ��У���ȼ�� Ѹ�����뼯��SO2�ļ���ƿ | ����������ɫ���̣���ƿ ���а�ɫ�������� | SO2���л�ԭ�� |
A. A B. B C. C D. D




