��Ŀ����
20����֪A��B��C��D��E��F��G��H��IΪԪ�����ڱ���ԭ���������������ǰ������Ԫ�أ�Aԭ�ӵ��������p����ϵĵ���������ǰһ���Ӳ����������A��B��C��D��E��F��G�ֱ�λ��ͬһ���ڣ�Cԭ��L������2�Գɵ��ӣ�D��E��F�ĺ�������Ų���ͬ�ļ����ӿ��γ�һ��E3FD6�����Ӿ���X��EG��HCΪ��������ͬ�����Ӿ��壮Iԭ��M��Ϊȫ����״̬���Һ����δ�ɶԵ���ֻ��һ�������������������ش��������⣺������ʱ���ö�Ӧ��Ԫ�ط��ű�ʾ����1��CԪ�����ڵ���������̬�⻯���У��е����H2O�������ʽ����ԭ��ˮ���Ӽ������������ǻ�������е㣼���ǻ�������ķе㣨���������������������ԭ�������ǻ��������γɷ���������������ǻ��������γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������ʹ�е�����
��2��BԪ������Ԫ�ؿ��γ�16e-��ij������û��������ƽ��ṹ������ṹʽΪH-N=N-H��1mol�÷����к��Цļ�����Ŀ3NA���м�����ĿNA��
��3��Ԫ��B��G���γɻ�����BG3�����ݼ۲���ӶԻ��������ж�BG3�Ŀռ乹��Ϊ�����Σ�������Bԭ�ӵ��ӻ���ʽΪsp3�ӻ����÷������ڼ��� ���ӣ�����ԡ��Ǽ��ԡ��������ľ��������Ƿ��Ӿ��壻BG3������ˮ��ԭ��NCl3Ϊ���Է��ӣ�H2OΪ���Է��ӣ�������������ԭ����NCl3������ˮ��
��4��G�����ֳ����IJ�ͬ��̬�������������ǿ������˳��ΪHClO4��HClO3��HClO2��HClO
��5��ijͬѧ�������й�IԪ�ص�ʵ��������ͼ��
I����$��_{��}^{��������ȼ}$��ɫ����$��_{��}^{����ˮ}$��ɫ��Һ$��_{��}^{����}$��ɫ����$��_{��}^{����}$����ɫ��Һ$��_{��}^{H_{2}S}$��ɫ����
д����ʵ��������γ�����ɫ��Һ����Ҫ�ɷֵĻ�ѧʽ[Cu��NH3��4]2+���������ӻ��ϼ�+2����λ��4��������Һ�м��������Ҵ�������������ɫ���壬�仯ѧʽΪ[Cu��NH3��4]SO4•H2O�����ڻ�ѧ���������Ӽ������ۼ�����λ����д���ۢܢݷ�Ӧ�����ӷ���ʽCu2++2NH3•H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��[Cu��NH3��4]2++H2S+2H2O=CuS��+2NH4++2NH3•H2O��
��6��I�����У��侧��Ķѻ���ʽ������Ϊ�����������ܶѻ���Iԭ�ӵ���λ��Ϊ12��һ��������Iԭ�ӵ���ĿΪ4�����������ͬ�ѻ���ʽ�Ľ�������Ag��Au���ռ�������Ϊ74%��д��������̣���д������I�ܵ����ԭ��ͭ�ǽ������壬�ɽ��������Ӻ����ɵ��ӹ��ɣ����ɵ�������ӵ糡�������¿ɷ��������ƶ���
���� ԭ�ӵ��������p����ϵĵ���������ǰһ���Ӳ����������ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����AΪ̼Ԫ�أ�Cԭ��L������2�ԳɶԵ��ӣ���������Ų�Ϊ1s2s22p4����CΪ��Ԫ�أ�A��B��C��Dԭ����������������ͬ���ڣ�����BΪ��Ԫ�أ���DΪ��Ԫ�أ�
D��E��F�ļ����Ӻ�������Ų���ͬ�����������Ϊ10����E��F���ڵ������ڣ����߿��γ�һ��E3FD6�����Ӿ���X����֪EΪ+1�ۡ�FΪ+3�ۣ���EΪNa��FΪAl��E��F��G�ֱ�λ��ͬһ���ڣ�EG��HCΪ��������ͬ�����Ӿ��壬��G����-1�ۡ�H����+2�ۣ�����֪GΪCl��HΪMg��Iԭ��M��Ϊȫ����״̬���Һ����δ�ɶԵ���ֻ��һ����ԭ�Ӻ��������Ϊ2+8+18+1=29����IΪCu��
��� �⣺ԭ�ӵ��������p����ϵĵ���������ǰһ���Ӳ����������ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����AΪ̼Ԫ�أ�Cԭ��L������2�ԳɶԵ��ӣ���������Ų�Ϊ1s2s22p4����CΪ��Ԫ�أ�A��B��C��Dԭ����������������ͬ���ڣ�����BΪ��Ԫ�أ���DΪ��Ԫ�أ�
D��E��F�ļ����Ӻ�������Ų���ͬ�����������Ϊ10����E��F���ڵ������ڣ����߿��γ�һ��E3FD6�����Ӿ���X����֪EΪ+1�ۡ�FΪ+3�ۣ���EΪNa��FΪAl��E��F��G�ֱ�λ��ͬһ���ڣ�EG��HCΪ��������ͬ�����Ӿ��壬��G����-1�ۡ�H����+2�ۣ�����֪GΪCl��HΪMg��Iԭ��M��Ϊȫ����״̬���Һ����δ�ɶԵ���ֻ��һ����ԭ�Ӻ��������Ϊ2+8+18+1=29����IΪCu��
��1��CΪOԪ�أ����ڵ���������̬�⻯���У�ˮ����֮�����������е���ߣ����ǻ��������γɷ���������������ǻ��������γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������ʹ�е����ߣ������ǻ�������е���ڶ��ǻ�������ķе㣬
�ʴ�Ϊ��H2O��ˮ���Ӽ����������������ǻ��������γɷ���������������ǻ��������γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������ʹ�е����ߣ�
��2��BΪNԪ�أ�����Ԫ�ؿ��γ�16e-��ij������û��������ƽ��ṹ���û�����N2H2��Nԭ��֮���γ�2�Թ��õ��Ӷԣ�Nԭ����Hԭ��֮���γ�1�Թ��õ��Ӷԣ���Nԭ�Ӻ���1�Թ¶Ե��ӣ�����ṹʽΪ H-N=N-H��1mol�÷����к��Цļ�����ĿΪ3NA���м�����ĿΪNA��
�ʴ�Ϊ��H-N=N-H��3NA��NA��
��3��Ԫ��B��G���γɻ�����BG3ΪNCl3��������Nԭ�Ӽ۲���Ӷ���Ϊ3+$\frac{5-1��3}{2}$=4����1�Թµ��Ӷԣ�����ռ乹��Ϊ�����Σ�������Nԭ�ӵ��ӻ���ʽΪsp3 �ӻ�������������������IJ��غϣ����ڼ��Է��ӣ����ľ��������Ƿ��Ӿ��壻NCl3Ϊ���Է��ӣ�H2OΪ���Է��ӣ�������������ԭ����NCl3������ˮ��
�ʴ�Ϊ�������Σ�sp3�����ԣ����ӣ�NCl3Ϊ���Է��ӣ�H2OΪ���Է��ӣ�������������ԭ����NCl3������ˮ��
��4��G�����ֳ����IJ�ͬ��̬������ΪHClO4��HClO3��HClO2��HClO�����ǻ�����Ŀ����Ϊ3��2��1��0�����ǻ���Խ�࣬����Խǿ����������ǿ������˳��ΪHClO4��HClO3��HClO2��HClO��
�ʴ�Ϊ��HClO4��HClO3��HClO2��HClO��
��5��Cu��������ȼ������CuCl2���Ȼ�ͭ�백ˮ��Ӧ����Cu��OH��2������������������ͭ�ܽ�����[Cu��NH3��4]2+�����ͨ�����⣬����CuS��ɫ������
��ʵ��������γ�����ɫ��Һ����Ҫ�ɷֵĻ�ѧʽΪ��[Cu��NH3��4]2+��ͭ����Ϊ�������ӣ����ϼ�Ϊ+2����λ��Ϊ4��������Һ�м��������Ҵ�������������ɫ���壬�仯ѧʽΪ[Cu��NH3��4]SO4•H2O�����ڻ�ѧ�������У����Ӽ������ۼ�����λ����
��Ӧ�۷�Ӧ�����ӷ���ʽΪ��Cu2++2NH3•H2O=Cu��OH��2��+2NH4+��
��Ӧ�ܷ�Ӧ�����ӷ���ʽΪ��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
��Ӧ�ݷ�Ӧ�����ӷ���ʽΪ��[Cu��NH3��4]2++H2S+2H2O=CuS��+2NH4++2NH3•H2O��
�ʴ�Ϊ��[Cu��NH3��4]2+��+2��4������ɫ��[Cu��NH3��4]SO4•H2O�����Ӽ������ۼ�����λ����Cu2++2NH3•H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��[Cu��NH3��4]2++H2S+2H2O=CuS��+2NH4++2NH3•H2O��
��6��Cu�����У��侧��Ķѻ���ʽ������Ϊ�������������ܶѻ���Cuԭ�ӵ���λ��Ϊ12��һ��������Cuԭ�ӵ���ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�����������ͬ�ѻ���ʽ�Ľ�������Ag��Au��
��Cuԭ�Ӱ뾶Ϊr������Cuԭ�������Ϊ4��$\frac{4}{3}$��r3�������ⳤΪ4r��$\frac{\sqrt{2}}{2}$=2$\sqrt{2}$r�������Ϊ��2$\sqrt{2}$r��3=16$\sqrt{2}$r3���ռ�������Ϊ[��4��$\frac{4}{3}$��r3����16$\sqrt{2}$r3]��100%��74%��
ͭ�ǽ������壬�ɽ��������Ӻ����ɵ��ӹ��ɣ����ɵ�������ӵ糡�������¿ɷ��������ƶ���Cu���Ե��磬
�ʴ�Ϊ�������������ܶѻ���12��4��Ag��Au��74%��ͭ�ǽ������壬�ɽ��������Ӻ����ɵ��ӹ��ɣ����ɵ�������ӵ糡�������¿ɷ��������ƶ���
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų�����������Ӽ��ԡ��ӻ�������ռ乹�͡���ѧ�������������ṹ�����ȣ������ʽṹ����֪ʶ���ۺϿ��飬��Ҫѧ���߱���ʵ�Ļ����������ѧ��������ṹ���Ѷ��еȣ�

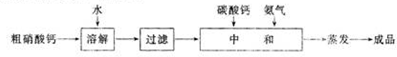
| A�� | ʹ�ô�װ�ÿ��Լ��ٵ��������������ŷ� | |
| B�� | ��װ���ڼȷ����˻��Ϸ�Ӧ��Ҳ�����˷ֽⷴӦ | |
| C�� | �ܷ�Ӧ�ɱ�ʾΪ��2SO2+2CaCO3+O2��2CaSO4+2CO2 | |
| D�� | ���ŷŵ�������ʹ����ʯ��ˮ����ǣ�˵���������к�SO2 |
| A�� | ���Ը��������Һ | B�� | Ũ��ˮ | ||
| C�� | �Ȼ�����Һ | D�� | ����������Һ |
| A�� | ���������� | B�� | ��Һ��Ca2+����Ŀ���� | ||
| C�� | �ܼ���������С | D�� | ��Һ��pH���� |
| A�� | �����£�CH3COONH4��Һ��pH=7���봿ˮ��H2O�ĵ���̶���ͬ | |
| B�� | ��CH3COONH4��Һ����CH3COONa����ʱ��c��NH4+����c��CH3COO-���������� | |
| C�� | �����£���Ũ�ȵ�NH4Cl��CH3COONa����Һ��pH֮��Ϊ14 | |
| D�� | ���µ�Ũ�ȵİ�ˮ�ʹ�������Һ��ˮϡ�͵���ͬ�������ҺpH�ı仯ֵһ����ͬ |
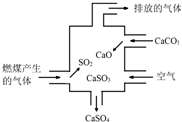
| A�� | Ϊ��ʹȼ�ϳ��ȼ�գ�ͨ��Ŀ���Ҫ�ʵ����� | |
| B�� | Ŀǰ��¯ȼ�ղ��÷���¯�������࣬Ŀ�������ú̿����Ч�ʲ�����SO2���ŷ� | |
| C�� | Ϊ������ܵ������ʣ�������úȼ�պ��ŷŷ������̵��а�װ�Ƚ���װ�� | |
| D�� | Ϊ�������ܣ����о��跨��̫���ܾ۽�����������ʹˮ�ֽ�������� |
| A�� | 0.1mol•L-1 HA ��c��H+��=c��OH-��+c��A-���� | |
| B�� | 0.1mol•L-1 HA �� 0.1 mol•L-1NaOH �������Һ�����ԣ�c��Na+����c��A-�� | |
| C�� | 0.1mol•L-1 NaA ��c��Na+����c��OH-����c��A-����c��H+�� | |
| D�� | 0.1mol•L-1 HA�м�������NaA���壬HA�ĵ��볣����С |
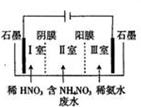 ����笠�[5Ca��NO3��2•NH4NO3•10H2O]��������ˮ����һ����ɫ���Ϸ��ϣ�
����笠�[5Ca��NO3��2•NH4NO3•10H2O]��������ˮ����һ����ɫ���Ϸ��ϣ�